VirB11 ATPases are dynamic hexameric assemblies: new insights into bacterial type IV secretion - PubMed (original) (raw)
VirB11 ATPases are dynamic hexameric assemblies: new insights into bacterial type IV secretion
Savvas N Savvides et al. EMBO J. 2003.
Abstract
The coupling of ATP binding/hydrolysis to macromolecular secretion systems is crucial to the pathogenicity of Gram-negative bacteria. We reported previously the structure of the ADP-bound form of the hexameric traffic VirB11 ATPase of the Helicobacter pylori type IV secretion system (named HP0525), and proposed that it functions as a gating molecule at the inner membrane, cycling through closed and open forms regulated by ATP binding/hydrolysis. Here, we combine crystal structures with analytical ultracentrifugation experiments to show that VirB11 ATPases indeed function as dynamic hexameric assemblies. In the absence of nucleotide, the N-terminal domains exhibit a collection of rigid-body conformations. Nucleotide binding 'locks' the hexamer into a symmetric and compact structure. We propose that VirB11s use the mechanical leverage generated by such nucleotide-dependent conformational changes to facilitate the export of substrates or the assembly of the type IV secretion apparatus. Biochemical characterization of mutant forms of HP0525 coupled with electron microscopy and in vivo assays support such hypothesis, and establish the relevance of VirB11s ATPases as drug targets against pathogenic bacteria.
Figures
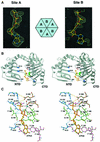
Fig. 1. Structure of apo-HP0525. (A) Representative region (the β1 strand of molecule A) of the experimental solvent-flattened electron density map at 3.0 Å resolution (contoured at 1.5σ) overlaid with the final refined model (Jones et al., 1991). (B) Stereo diagram of the structural superposition of apo-HP0525 (violet) and the ADP–HP0525 complex (gray) (Carson, 1997). The notation A–F is arbitrary, with molecule A defined as the first subunit listed in the PDB coordinate files of the unbound and ATPγS-bound structures. The two hexameric assemblies were overlaid with respect to molecule A of ADP–HP0525. Molecule F exhibits the largest conformational change and is colored green (apo-HP0525) and red (ADP–HP0525) in apo-HP0525 and ADP–HP0525, respectively. (C) Side view of the hexameric assemblies. This orientation is obtained by rotating the molecules shown in (B) 90° anti-clockwise along the vertical axis of (B). This view documents the extent of the conformational change in subunit F. (D) Structural variability between individual subunits in apo-HP0525. The overlay was carried out with respect to the CTD of subunit A of ADP–HP0525 (red). Subunit F of apo-HP0525 (green) exhibits the largest rotation (∼15° more open) about the hinge region between the NTD and CTD. The coloring scheme for all other subunits is as follows: apo-HP0525_A, dark gray; apo-HP0525_B, pink; apo-HP0525_C, orange; apo-HP0525_D, yellow; apo-HP0525_E, cyan.
Fig. 2. Structure of the ATPγS–HP0525 complex. (A) Simulated annealing omit _F_o–_F_c electron density maps contoured at 3σ, illustrating the relative binding of ATPγS in molecules A and B of the ATPγS–HP0525 complex (Jones et al., 1991). The β-phosphate position of ATPγS in site B is co-occupied by a sulfate ion. (B) Stereo diagram of subunit A of the ATPγS–HP0525 complex with the various components of the ATPγS binding site mapped onto the tertiary structure (Carson, 1997). ATPγS is colored in gold, while regions of the subunit interacting with the nucleotide are shown as follows: NTD residues (blue), P-loop (green), the putative catalytic Glu248 and Glu209 (red), CTD residues other than Glu248, Glu209 and P-loop residues (purple). (C) Stereo diagram of the ATPγS binding site in molecule A (site A) of the ATPγS–HP0525 complex. Coordination of a magnesium ion by ATPγS, the protein and a water molecule is shown in black dashed lines. Hydrogen bonds between HP0525 and ATPγS are shown in cyan dashed lines, while orange dashed lines illustrate the bifurcated hydrogen bonding interactions of Arg113 and His273 with the proposed catalytic Glu248 (see main text). For consistency, this view and the color coding of bonds are the same as in (B).
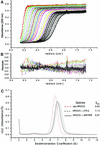
Fig. 3. Sedimentation coefficient distribution analysis of apo-, ATPγS-bound and AMP-PNP-bound HP0525. (A) Sedimentation velocity data of 5 µM HP0525 centrifuged at 40 000 r.p.m. Lines represent linear fits of the continuous c(s) conformational change model when constraining the molecular mass to 225 kDa, as a model for the sedimentation coefficient distribution of hexameric HP0525. Different colors are used to distinguish between successive data collection times. (B) Residuals from the linear fits described in (A). (C) Sedimentation coefficient distributions c(s) from the conformational change model.
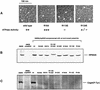
Fig. 4. Biochemical properties of HP0525 mutant proteins in H.pylori and test of their function in a CagA translocation assay. (A) Electron micrographs of negatively stained HP0525 proteins. Samples were treated as described in Krause et al. (2000b). Hexameric particles are indicated by white arrowheads. (B) Stable production of HP0525 mutant proteins after complementation of the 26695Δ_hp0525_ mutant strain with the mutant genes. (C) HP0525-dependent transport of CagA into gastric epithelial cells.
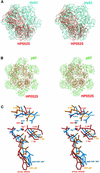
Fig. 5. Similarity of HP0525 to p97 (Carson, 1997). (A) Stereo diagram of a structural comparison between ADP–HP0525 (red) and ADP-bound HslU (cyan, PDB code: 1G41). The superposition is with respect to P-loop residues for one equivalent subunit from each hexamer. This figure illustrates the lack of similarity between the hexameric assembly of HP0525 and that of HslU (shown here), RecA, T7 gene 4 helicase and TrwB. (B) Stereo diagram of a structural comparison between ADP–HP0525 (red) and ADP-bound p97 (green, PDB code: 1E32), superimposed as in (A). p97 (shown here) and NSF are the only hexameric ATPases that have a hexameric assembly similar to HP0525. (C) Stereo diagram showing the structural mimicry of the DExx box motif by VirB11 ATPases, in comparison with p97 and NSF. The nucleotides and the residues of the DExx boxes are shown in ball-and-stick representation color-coded blue for the AMP-PNP –bound NSF, orange for the ADP-bound p97 and red for the ATPγS-bound HP0525.
Fig. 6. Model for the mode of action of VirB11 ATPases. The N-terminal and C-terminal domains are represented in pink and light blue, respectively. NTDs locked in a rigid conformation by the binding of ATP and ADP are shown in cyan and yellow, respectively.
Similar articles
- Crystal structure of the hexameric traffic ATPase of the Helicobacter pylori type IV secretion system.
Yeo HJ, Savvides SN, Herr AB, Lanka E, Waksman G. Yeo HJ, et al. Mol Cell. 2000 Dec;6(6):1461-72. doi: 10.1016/s1097-2765(00)00142-8. Mol Cell. 2000. PMID: 11163218 - A large domain swap in the VirB11 ATPase of Brucella suis leaves the hexameric assembly intact.
Hare S, Bayliss R, Baron C, Waksman G. Hare S, et al. J Mol Biol. 2006 Jun 30;360(1):56-66. doi: 10.1016/j.jmb.2006.04.060. Epub 2006 May 11. J Mol Biol. 2006. PMID: 16730027 - Identification, structure and mode of action of a new regulator of the Helicobacter pylori HP0525 ATPase.
Hare S, Fischer W, Williams R, Terradot L, Bayliss R, Haas R, Waksman G. Hare S, et al. EMBO J. 2007 Nov 28;26(23):4926-34. doi: 10.1038/sj.emboj.7601904. Epub 2007 Nov 1. EMBO J. 2007. PMID: 17972918 Free PMC article. - Bacterial transition metal P(1B)-ATPases: transport mechanism and roles in virulence.
Argüello JM, González-Guerrero M, Raimunda D. Argüello JM, et al. Biochemistry. 2011 Nov 22;50(46):9940-9. doi: 10.1021/bi201418k. Epub 2011 Oct 31. Biochemistry. 2011. PMID: 21999638 Free PMC article. Review. - The structural biology of type IV secretion systems.
Fronzes R, Christie PJ, Waksman G. Fronzes R, et al. Nat Rev Microbiol. 2009 Oct;7(10):703-14. doi: 10.1038/nrmicro2218. Nat Rev Microbiol. 2009. PMID: 19756009 Free PMC article. Review.
Cited by
- Evidence for VirB4-mediated dislocation of membrane-integrated VirB2 pilin during biogenesis of the Agrobacterium VirB/VirD4 type IV secretion system.
Kerr JE, Christie PJ. Kerr JE, et al. J Bacteriol. 2010 Oct;192(19):4923-34. doi: 10.1128/JB.00557-10. Epub 2010 Jul 23. J Bacteriol. 2010. PMID: 20656905 Free PMC article. - Interaction between protein subunits of the type IV secretion system of Bartonella henselae.
Shamaei-Tousi A, Cahill R, Frankel G. Shamaei-Tousi A, et al. J Bacteriol. 2004 Jul;186(14):4796-801. doi: 10.1128/JB.186.14.4796-4801.2004. J Bacteriol. 2004. PMID: 15231811 Free PMC article. - Process of protein transport by the type III secretion system.
Ghosh P. Ghosh P. Microbiol Mol Biol Rev. 2004 Dec;68(4):771-95. doi: 10.1128/MMBR.68.4.771-795.2004. Microbiol Mol Biol Rev. 2004. PMID: 15590783 Free PMC article. Review. - Toxins from bacteria.
Henkel JS, Baldwin MR, Barbieri JT. Henkel JS, et al. EXS. 2010;100:1-29. doi: 10.1007/978-3-7643-8338-1_1. EXS. 2010. PMID: 20358680 Free PMC article. Review. - Structural organisation of the type IV secretion systems.
Waksman G, Orlova EV. Waksman G, et al. Curr Opin Microbiol. 2014 Feb;17(100):24-31. doi: 10.1016/j.mib.2013.11.001. Epub 2013 Dec 5. Curr Opin Microbiol. 2014. PMID: 24581689 Free PMC article. Review.
References
- Abraham J.P., Leslie,A.G.W., Lutter,R. and Walker,J.E. (1994) Structure at 2.8 Å resolution of F1-ATPase from bovine heart mitochondria. Nature, 370, 621–628. - PubMed
- Backert S., Ziska,E., Brinkmann,V.Z.-A.U., Fauconnier,A., Jungblut,P.R., Naumann,M. and Meyer,T.F. (2000) Translocation of the Helicobacter pylori CagA protein in gastric epithelial cells by a type IV secretion apparatus. Cell. Microbiol., 2, 155–164. - PubMed
- Brünger A.T. et al. (1998) Crystallography and NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D, 54, 905–921. - PubMed
- Carson M. (1997) Ribbons. Methods Enzymol., 277, 493–505. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources