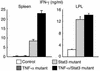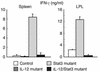Toll-like receptor-dependent production of IL-12p40 causes chronic enterocolitis in myeloid cell-specific Stat3-deficient mice - PubMed (original) (raw)
Toll-like receptor-dependent production of IL-12p40 causes chronic enterocolitis in myeloid cell-specific Stat3-deficient mice
Masaya Kobayashi et al. J Clin Invest. 2003 May.
Abstract
Stat3 plays an essential role in IL-10 signaling pathways. A myeloid cell-specific deletion of Stat3 resulted in inflammatory cytokine production and development of chronic enterocolitis with enhanced Th1 responses in mice. In this study, we analyzed the mechanism by which a Stat3 deficiency in myeloid cells led to the induction of chronic enterocolitis in vivo. Even in the absence of Stat1, which is essential for IFN-gamma signaling pathways, Stat3 mutant mice developed chronic enterocolitis. TNF-alpha/Stat3 double-mutant mice developed severe chronic enterocolitis with enhanced Th1 cell development. IL-12p40/Stat3 double-mutant mice, however, showed normal Th1 responses and no inflammatory change in the colon. RAG2/Stat3 double-mutant mice did not develop enterocolitis, either. These findings indicate that overproduction of IL-12p40, which induces potent Th1 responses, is essential for the development of chronic enterocolitis in Stat3 mutant mice. Furthermore, enterocolitis was significantly improved and IFN-gamma production by T cells was reduced in TLR4/Stat3 double-mutant mice, indicating that TLR4-mediated recognition of microbial components triggers aberrant IL-12p40 production by myeloid cells, leading to the development of enterocolitis. Thus, this study clearly established a sequential innate and acquired immune mechanism for the development of Th1-dependent enterocolitis.
Figures
Figure 1
IBDs in Stat1/Stat3 double-deficient mice. (a-d) Histologic examination of the colons of (a) control, (b) Stat1-deficient, (c) Stat3 mutant, and (d) Stat1/Stat3 double-mutant mice at 8 weeks. H&E staining is shown. ×20. (e)The colitis scores shown for individual mice at 16 weeks of age were total scores for individual sections as described in Methods. The severity of colitis in Stat1/Stat3 double-mutant mice was significantly but only partially improved compared with Stat3 mutant mice (**P < 0.01). *P < 0.001 versus group of control mice.
Figure 2
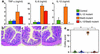
Enhanced IFN-γ production in Stat1/Stat3 double-deficient mice. (a) Spleen cells were stimulated with PMA and ionomycin for 6 h, then stained for CD4, fixed, and finally stained for IFN-γ and IL-4. Cells were analyzed on FACS by gating on CD4+ population. The frequency of cytokine-producing CD4+ cells is indicated as a percentage. (b) CD4+ T cells were purified from lamina propria and stimulated with plate-bound anti-CD3 Ab. The concentration of IFN-γ was measured by ELISA. (c) Mice were intraperitoneally injected with thioglycollate, and 3 days later peritoneal macrophages were isolated. Macrophages were stimulated with 10 ng/ml LPS for 24 h. Concentrations of TNF-α, IL-6, and IL-12p40 in the culture supernatants were measured.
Figure 3
IBDs in TNF-α/Stat3 double-deficient mice. (a–d) Histologic examination of the colons of (a) control, (b) TNF-α–deficient, (c) Stat3 mutant, and (d) TNF-α/Stat3 double-mutant mice at 16 weeks. H&E staining is shown. ×20. (e) The colitis score of TNF-α/Stat3 double-mutant mice at 16 weeks of age. TNF-α/Stat3 double-mutant mice developed severe chronic colitis, similar to Stat3 mutant mice. *P < 0.001 versus group of control mice. (f) Mice were intraperitoneally injected with thioglycollate, and 3 days later peritoneal macrophages were isolated. Macrophages were stimulated with 10 ng/ml LPS for 24 h. Concentrations of TNF-α, IL-6, and IL-12p40 in the culture supernatants were measured. *Not detected.
Figure 4
IFN-γ production by CD4+ T cells in spleen and lamina propria of TNF-α/Stat3 double-mutant mice. CD4+ T cells were purified from spleen and lamina propria (LPL), and stimulated with plate-bound anti-CD3 Ab. The concentration of IFN-γ was measured by ELISA. CD4+ T cells from TNF-α/Stat3 double-mutant mice produced significantly increased levels of IFN-γ, similar to Stat3 mutant mice.
Figure 5
IL-12/Stat3 double-mutant mice showed no inflammatory change in the colon. (a) Peritoneal macrophages were stimulated with 10 ng/ml LPS for 24 h. Concentrations of TNF-α, IL-6, and IL-12p40 in the culture supernatants were measured. *Not detected. (b–d) Histopathology of the colons of (b) control, (c) IL-12–deficient, and (d) IL-12/Stat3 double-mutant mice at 16 weeks. H&E staining is shown. ×20. (e) The colitis score of IL-12/Stat3 double-mutant mice at 16 weeks of age. None of the IL-12/Stat3 double-mutant mice showed any inflammatory change in the colon. *P < 0.001 between Stat3 mutant mice and control or IL-12/Stat3 double-mutant mice.
Figure 6
Normal production of IFN-γ by CD4+ T cells in spleen and lamina propria of IL-12/Stat3 double-mutant mice. CD4+ T cells were purified from spleen and lamina propria (LPL) and stimulated with plate-bound anti-CD3 Ab. The concentration of IFN-γ was measured by ELISA. CD4+ T cells from IL-12/Stat3 double-mutant mice produced normal levels of IFN-γ, unlike those from Stat3 mutant mice.
Figure 7
RAG2/Stat3 double-mutant mice showed no inflammatory change in the colon. (a) Peritoneal macrophages were stimulated with 10 ng/ml LPS for 24 h. Concentrations of TNF-α, IL-6, and IL-12p40 in the culture supernatants were measured. (b–d) Histopathology of the colon of (b) control, (c) RAG2-deficient, and (d) RAG2/Stat3 double-mutant mice at 16 weeks. H&E staining is shown. ×20. (e) The colitis score of RAG2/Stat3 double-mutant mice at 16 weeks of age. None of the RAG2/Stat3 double-mutant mice showed any inflammatory change in the colon. *P < 0.001 between Stat3 mutant mice and control or RAG2/Stat3 double-mutant mice.
Figure 8
Improved colitis in TLR4/Stat3 double-mutant mice. (a) Peritoneal macrophages were stimulated with 10 ng/ml LPS for 24 h. Concentrations of TNF-α, IL-6, and IL-12p40 in the culture supernatants were measured. LPS-induced production of inflammatory cytokines was abolished in TLR4/Stat3 double-mutant macrophages. *Not detected. (b) CD4+ T cells were purified from spleen and stimulated with plate-bound anti-CD3 Ab. The concentration of IFN-γ was measured by ELISA. CD4+ T cells from TLR4/Stat3 double-mutant mice produced reduced levels of IFN-γ compared with Stat3 mutant mice. (c–e) Histopathology of the colon of (c) control, (d) TLR4-deficient, and (e) TLR4/Stat3 double-mutant mice at 16 weeks. H&E staining is shown. ×20. (f) The colitis score of TLR4/Stat3 double-mutant mice at 16 weeks of age. TLR4/Stat3 double-mutant mice showed no or moderate inflammatory changes in the colon. *P < 0.001 between Stat3 mutant mice and control or TLR4/Stat3 double-mutant mice.
Figure 9
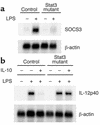
Impaired IL-10–induced expression of SOCS3 in Stat3 mutant macrophages. (a) Peritoneal macrophages from control and Stat3 mutant mice were stimulated with 10 ng/ml IL-10 for 2 h and analyzed for SOCS3 mRNA expression by Northern blotting. (b) Peritoneal macrophages from control and Stat3 mutant mice were cultured in the presence or absence of 10 ng/ml IL-10 for 24 h and then stimulated with 10 ng/ml LPS for 2 h. Total RNA was extracted and subjected to Northern blot analysis for IL-12p40 mRNA expression. The same membrane was rehybridized with β-actin.
Figure 10
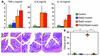
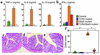
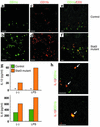
Macrophages and dendritic cells in the large intestine were increased in number and produced IL-12p40 in Stat3 mutant mice with colitis. (a–f) Immunohistochemistry of the colon of (a, c, e) control and (b, d, f) Stat3 mutant mice at 16 weeks. The colon sections were stained with anti-CD11c Ab (green) (a, b, e, f), anti-CD11b Ab (red) (c and d), and anti-CD3 Ab (red) (e and f). Bar: 10 μm. (g) CD11c-positive dendritic cells in the large intestine were stimulated with 100 ng/ml LPS for 24 h. Concentrations of IL-6 and IL-12p40 in the culture supernatants were measured. *Not detected. (h) Immunohistochemistry of the colon of Stat3 mutant mice. The colon sections were stained with anti–IL-12p40 Ab (red) and anti-CD11c Ab (green) oranti-CD11b Ab (green). Bar: 10 μm.
Comment in
- Connecting the dots from Toll-like receptors to innate immune cells and inflammatory bowel disease.
Boone DL, Ma A. Boone DL, et al. J Clin Invest. 2003 May;111(9):1284-6. doi: 10.1172/JCI18545. J Clin Invest. 2003. PMID: 12727919 Free PMC article. No abstract available.
Similar articles
- STAT3 regulates NF-kappaB recruitment to the IL-12p40 promoter in dendritic cells.
Hoentjen F, Sartor RB, Ozaki M, Jobin C. Hoentjen F, et al. Blood. 2005 Jan 15;105(2):689-96. doi: 10.1182/blood-2004-04-1309. Epub 2004 Jul 13. Blood. 2005. PMID: 15251981 - IL-21 enhances SOCS gene expression and inhibits LPS-induced cytokine production in human monocyte-derived dendritic cells.
Strengell M, Lehtonen A, Matikainen S, Julkunen I. Strengell M, et al. J Leukoc Biol. 2006 Jun;79(6):1279-85. doi: 10.1189/jlb.0905503. Epub 2006 Mar 21. J Leukoc Biol. 2006. PMID: 16551679 - Opposing roles of STAT1 and STAT3 in T cell-mediated hepatitis: regulation by SOCS.
Hong F, Jaruga B, Kim WH, Radaeva S, El-Assal ON, Tian Z, Nguyen VA, Gao B. Hong F, et al. J Clin Invest. 2002 Nov;110(10):1503-13. doi: 10.1172/JCI15841. J Clin Invest. 2002. PMID: 12438448 Free PMC article. - Inactivation of Stat3 in tumor cells: releasing a brake on immune responses against cancer?
Gamero AM, Young HA, Wiltrout RH. Gamero AM, et al. Cancer Cell. 2004 Feb;5(2):111-2. doi: 10.1016/s1535-6108(04)00028-5. Cancer Cell. 2004. PMID: 14998485 Review. - SOCS3 in immune regulation of inflammatory bowel disease and inflammatory bowel disease-related cancer.
Li Y, de Haar C, Peppelenbosch MP, van der Woude CJ. Li Y, et al. Cytokine Growth Factor Rev. 2012 Jun;23(3):127-38. doi: 10.1016/j.cytogfr.2012.04.005. Epub 2012 May 15. Cytokine Growth Factor Rev. 2012. PMID: 22591635 Review.
Cited by
- The Complex Role of STAT3 in Viral Infections.
Kuchipudi SV. Kuchipudi SV. J Immunol Res. 2015;2015:272359. doi: 10.1155/2015/272359. Epub 2015 Jun 25. J Immunol Res. 2015. PMID: 26199948 Free PMC article. Review. - MyD88 signalling in colonic mononuclear phagocytes drives colitis in IL-10-deficient mice.
Hoshi N, Schenten D, Nish SA, Walther Z, Gagliani N, Flavell RA, Reizis B, Shen Z, Fox JG, Iwasaki A, Medzhitov R. Hoshi N, et al. Nat Commun. 2012;3:1120. doi: 10.1038/ncomms2113. Nat Commun. 2012. PMID: 23047678 Free PMC article. - Toll-like receptor (TLR) 4 polymorphisms are associated with a chronic course of sarcoidosis.
Pabst S, Baumgarten G, Stremmel A, Lennarz M, Knüfermann P, Gillissen A, Vetter H, Grohé C. Pabst S, et al. Clin Exp Immunol. 2006 Mar;143(3):420-6. doi: 10.1111/j.1365-2249.2006.03008.x. Clin Exp Immunol. 2006. PMID: 16487240 Free PMC article. - Gatekeepers of intestinal inflammation.
Arnett HA, Viney JL. Arnett HA, et al. Inflamm Res. 2010 Jan;59(1):1-14. doi: 10.1007/s00011-009-0091-x. Inflamm Res. 2010. PMID: 20066780 Review. - STAT3 regulates proliferation and survival of CD8+ T cells: enhances effector responses to HSV-1 infection, and inhibits IL-10+ regulatory CD8+ T cells in autoimmune uveitis.
Yu CR, Dambuza IM, Lee YJ, Frank GM, Egwuagu CE. Yu CR, et al. Mediators Inflamm. 2013;2013:359674. doi: 10.1155/2013/359674. Epub 2013 Sep 24. Mediators Inflamm. 2013. PMID: 24204098 Free PMC article.
References
- Podolsky DK. Inflammatory bowel disease. N. Engl. J. Med. 1991;325:928–937. - PubMed
- Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. - PubMed
- Mombaerts P, et al. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell. 1993;75:274–282. - PubMed
- Wirtz S, et al. Cutting edge: Chronic intestinal inflammation in STAT-4 transgenic mice: characterization of disease and adoptive transfer by TNF- plus IFN-γ-producing CD4+ T cells that respond to bacterial antigens. J. Immunol. 1999;162:1884–1888. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous