Attenuation of Mycobacterium tuberculosis by disruption of a mas-like gene or a chalcone synthase-like gene, which causes deficiency in dimycocerosyl phthiocerol synthesis - PubMed (original) (raw)
Attenuation of Mycobacterium tuberculosis by disruption of a mas-like gene or a chalcone synthase-like gene, which causes deficiency in dimycocerosyl phthiocerol synthesis
Tatiana D Sirakova et al. J Bacteriol. 2003 May.
Abstract
Tuberculosis is one of the leading preventable causes of death. Emergence of drug-resistant tuberculosis makes the discovery of new targets for antimycobacterial drugs critical. The unique mycobacterial cell wall lipids are known to play an important role in pathogenesis, and therefore the genes responsible for their biosynthesis offer potential new targets. To assess the possible role of some of the genes potentially involved in cell wall lipid synthesis, we disrupted a mas-like gene, msl7, and a chalcone synthase-like gene, pks10, with phage-mediated delivery of the disruption construct, in which the target gene was disrupted by replacement of an internal segment with the hygromycin resistance gene (hyg). Gene disruption by allelic exchange in the case of each disruptant was confirmed by PCR and Southern blot analyses. Neither msl7 nor pks10 mutants could produce dimycocerosyl phthiocerol, although both could produce mycocerosic acids. Thus, it is concluded that these gene products are involved in the biosynthesis of phthiocerol. Both mutants were found to be attenuated in a murine model, supporting the hypothesis that dimycocerosyl phthiocerol is a virulence factor and thus the many steps involved in its biosynthesis offer potential novel targets for antimycobacterial therapy.
Figures
FIG. 1.
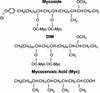
Structure of dimycocerosyl esters of glycosylated (Gl) phenolphthiocerol (mycoside) and phthiocerol (DIM).
FIG. 2.
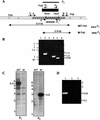
Disruption strategy for msl7 and evidence for gene replacement in the M. tuberculosis H37Rv mutant used for making the disruption construct. (A) Schematic representation of the construct used for disruption. Hatched, coding sequence; checkered, internal segment that was replaced with hyg gene cassette (black box); unshaded, regions of the gene outside those used to make the disruption construct. Primers A and B were used to amplify the DNA segment used to generate the disruption construct. WT _Pst_I, _Pst_I fragment from the wild type, expected to hybridize with probe P1, representing the fragment deleted in making the construct. M _Pst_I, _Pst_I fragment form the msl7 mutant, expected to hybridize with probe P2 (hyg gene). (B) PCR analysis of msl7 mutant. Lanes 1 and 2, PCR analysis of the 5′-flanking region, showing the product expected from the gene replacement mutant in lane 2 but not in the wild type (lane 1); primers C and H1. Lanes 3 and 4, PCR analysis of the 3′-flanking region, showing the expected product from the msl7 mutant (lane 4) but not from the wild type (lane 3); primers H2 and D. Lanes 5 and 6 show the PCR product representing the internal segment replaced by hyg from the wild type (lane 5) but not from the msl7 mutant (lane 6); primers E and F. (C) Southern blot analysis of M. tuberculosis H37Rv and the msl7 mutant. Genomic DNA was digested with _Pst_I and hybridized with probe P1 (left) or P2 (right). WT, wild type; M, mutant. (D) Reverse transcription-PCR showing the presence of transcripts containing the pks1 segment of msl7 in M. tuberculosis H37Rv. For lanes 1, 2, and 3, primers E and F were used. Lane 1, wild type; lane 2, wild type without reverse transcriptase; lane 3, msl7 mutant. In all panels, sizes are shown in kilobases.
FIG. 3.
Disruption strategy for pks10 and evidence for pks10 gene replacement in M. tuberculosis H37Rv. (A) Schematic representation of the disruption construct. Hatched and checkered regions represent the region used to make the disruption construct (amplified with primers A and B). The checkered segment was replaced with the hyg gene cassette (black box). Primer pairs C/H1, D/H2, and E/F were used for PCR analysis of homologous recombination as described in the text. WT _Pst_I, WT _Bam_HI, M _Pst_I, and M _Bam_HI are _Pst_I and _Bam_HI fragments from the wild-type (WT) and pks10 mutant (M) that are expected to hybridize with probes P1, representing the fragment deleted in making the construct, and P2, the hygromycin resistance gene cassette, respectively. (B) PCR analysis of 5′-flanking (lanes 1 and 2, primers C and H1), 3′-flanking (lanes 3 and 4, primers H2 and D), and the segment replaced by hyg (lane 5 and 6, primers E and F), demonstrating homologous recombination. Lanes 1, 3, and 5, wild type; lanes 2, 4, and 6, mutant. (C) Reverse transcription-PCR analysis showing expression of pks10 in the wild type (right) but not in the pks10 mutant (left). Primers, E and F. (D) Southern blot analysis of M. tuberculosis H37Rv and pks10 mutants. Genomic DNA was digested with _Pst_I and _Bam_HI. Left, hybridized with pks10 segment replaced by hyg (probe P1); right, probed with hyg gene (probe P2). WT, wild type; M1 and M2, mutants.
FIG. 4.
Autoradiogram of thin-layer chromatogram of lipids derived from sodium [1-14C]propionate in M. tuberculosis H37Rv (wild type [WT]), msl7 mutant, and the pks10 mutant. Total lipids were subjected to TLC on silica gel G with 10% ethyl ether in _n_-hexane as the solvent. A similar amount of radioactivity was used in each case. DIM, dimycocerosyl phthiocerol.
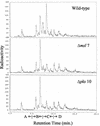
FIG. 5.
Radio-GC analysis of total fatty acid methyl esters derived from sodium [1-14C]propionate in M. tuberculosis H37Rv (wild type), the msl7 mutant, and the pks10 mutant. Retention time ranges for branched short-chain fatty acids (A), mycolipanoic and mycolipenic acids (B), mycocerosic acids (C), and phthioceranic acids (D) are indicated.
FIG. 6.
Growth of intranasally administered M. tuberculosis H37Rv and its msl7 and pks10 gene-disrupted mutants in the lungs of C57BL/6J mice. The experimental details are in the text.
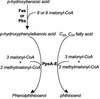
FIG. 7.
Major stages involved in the biosynthesis of phenolphthiocerol and phthiocerol. CoA, coenzyme A.
Similar articles
- The largest open reading frame (pks12) in the Mycobacterium tuberculosis genome is involved in pathogenesis and dimycocerosyl phthiocerol synthesis.
Sirakova TD, Dubey VS, Kim HJ, Cynamon MH, Kolattukudy PE. Sirakova TD, et al. Infect Immun. 2003 Jul;71(7):3794-801. doi: 10.1128/IAI.71.7.3794-3801.2003. Infect Immun. 2003. PMID: 12819062 Free PMC article. - Regulation of expression of mas and fadD28, two genes involved in production of dimycocerosyl phthiocerol, a virulence factor of Mycobacterium tuberculosis.
Sirakova TD, Fitzmaurice AM, Kolattukudy P. Sirakova TD, et al. J Bacteriol. 2002 Dec;184(24):6796-802. doi: 10.1128/JB.184.24.6796-6802.2002. J Bacteriol. 2002. PMID: 12446629 Free PMC article. - Biochemical function of msl5 (pks8 plus pks17) in Mycobacterium tuberculosis H37Rv: biosynthesis of monomethyl branched unsaturated fatty acids.
Dubey VS, Sirakova TD, Cynamon MH, Kolattukudy PE. Dubey VS, et al. J Bacteriol. 2003 Aug;185(15):4620-5. doi: 10.1128/JB.185.15.4620-4625.2003. J Bacteriol. 2003. PMID: 12867474 Free PMC article. - Mycobacterium bovis lipids: virulence and vaccines.
Hotter GS, Collins DM. Hotter GS, et al. Vet Microbiol. 2011 Jul 5;151(1-2):91-8. doi: 10.1016/j.vetmic.2011.02.030. Epub 2011 Feb 24. Vet Microbiol. 2011. PMID: 21420803 Review. - Lipid transport in Mycobacterium tuberculosis and its implications in virulence and drug development.
Bailo R, Bhatt A, Aínsa JA. Bailo R, et al. Biochem Pharmacol. 2015 Aug 1;96(3):159-67. doi: 10.1016/j.bcp.2015.05.001. Epub 2015 May 16. Biochem Pharmacol. 2015. PMID: 25986884 Review.
Cited by
- Defining mycobacteria: Shared and specific genome features for different lifestyles.
Vissa VD, Sakamuri RM, Li W, Brennan PJ. Vissa VD, et al. Indian J Microbiol. 2009 Mar;49(1):11-47. doi: 10.1007/s12088-009-0006-0. Epub 2009 Feb 5. Indian J Microbiol. 2009. PMID: 23100749 Free PMC article. - The Mycobacterium tuberculosis extracytoplasmic-function sigma factor SigL regulates polyketide synthases and secreted or membrane proteins and is required for virulence.
Hahn MY, Raman S, Anaya M, Husson RN. Hahn MY, et al. J Bacteriol. 2005 Oct;187(20):7062-71. doi: 10.1128/JB.187.20.7062-7071.2005. J Bacteriol. 2005. PMID: 16199577 Free PMC article. - Genome Mining and Evolutionary Analysis Reveal Diverse Type III Polyketide Synthase Pathways in Cyanobacteria.
Larsen JS, Pearson LA, Neilan BA. Larsen JS, et al. Genome Biol Evol. 2021 Apr 5;13(4):evab056. doi: 10.1093/gbe/evab056. Genome Biol Evol. 2021. PMID: 33739400 Free PMC article. - Posttranslational regulation of Mycobacterium tuberculosis extracytoplasmic-function sigma factor sigma L and roles in virulence and in global regulation of gene expression.
Dainese E, Rodrigue S, Delogu G, Provvedi R, Laflamme L, Brzezinski R, Fadda G, Smith I, Gaudreau L, Palù G, Manganelli R. Dainese E, et al. Infect Immun. 2006 Apr;74(4):2457-61. doi: 10.1128/IAI.74.4.2457-2461.2006. Infect Immun. 2006. PMID: 16552079 Free PMC article. - The complete genome sequence of Mycobacterium avium subspecies paratuberculosis.
Li L, Bannantine JP, Zhang Q, Amonsin A, May BJ, Alt D, Banerji N, Kanjilal S, Kapur V. Li L, et al. Proc Natl Acad Sci U S A. 2005 Aug 30;102(35):12344-9. doi: 10.1073/pnas.0505662102. Epub 2005 Aug 22. Proc Natl Acad Sci U S A. 2005. PMID: 16116077 Free PMC article.
References
- Azad, A. K., T. D. Sirakova, N. D. Fernandes, and P. E. Kolattukudy. 1997. Gene knock-out reveals a novel gene cluster for the synthesis of a class of cell wall lipids unique to pathogenic mycobacteria. J. Biol. Chem. 272:16741-16745. - PubMed
- Betts, J. C., P. T. Lukey, L. C. Robb, R. A. McAdam, and K. Duncan. 2002. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol. Microbiol. 43:717-731. - PubMed
- Brennan, P. J., and H. Nikaido. 1995. The envelope of mycobacteria. Annu. Rev. Biochem. 64:29-63. - PubMed
- Camacho, L. R., D. Enserqueix, E. Perez, B. Gicquel, and C. Guilhot. 1999. Identification of a virulence gene cluster of Mycobacterium tuberculosis by signature-tagged transposon mutagenesis. Mol. Microbiol. 34:257-267. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases