Distinct response to dioxin in an arylhydrocarbon receptor (AHR)-humanized mouse - PubMed (original) (raw)
Distinct response to dioxin in an arylhydrocarbon receptor (AHR)-humanized mouse
Takashi Moriguchi et al. Proc Natl Acad Sci U S A. 2003.
Abstract
There are large inter- and intraspecies differences in susceptibility to dioxin-induced toxicities. A critical question in risk assessment of dioxin and related compounds is whether humans are sensitive or resistant to their toxicities. The diverse responses of mammals to dioxin are strongly influenced by functional polymorphisms of the arylhydrocarbon receptor (AHR). To characterize responses mediated by the human AHR (hAHR), we generated a mouse possessing hAHR instead of mouse AHR. Responses of these mice to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and 3-methylcholanthrene were compared with the responses of naturally sensitive (C57BL6J) and resistant (DBA2) mice. Mice homozygous for hAHR exhibited weaker induction of AHR target genes such as cyp1a1 and cyp1a2 than did C57BL6J (Ahr(b-1/b-1)) mice. DBA2 (Ahr(d/d)) mice were less responsive to induction of cyp genes than C57BL6J mice. hAHR and DBA2 AHR exhibit similar ligand-binding affinities and homozygous hAHR and Ahr(d/d) mice displayed comparable induction of AHR target genes by 3-methylcholanthrene. However, when TCDD was administered, a greatly diminished response was observed in homozygous hAHR mice compared with Ahr(d/d) mice, indicating that hAHR expressed in mice is functionally less responsive to TCDD than DBA2 AHR. After maternal exposure to TCDD, homozygous hAHR fetuses developed embryonic hydronephrosis, but not cleft palate, whereas fetuses possessing Ahr(b-1) or Ahr(d) developed both anomalies. These results suggest that hAHR may define the specificity of the responses to various AHR ligands. Thus, the hAHR knock-in mouse is a humanized model mouse that may better predict the biological effects of bioaccumulative environmental toxicants like TCDD in humans.
Figures
Figure 1
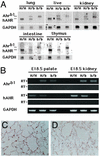
Generation of the hAHR knock-in mouse. (A) Strategy for hAHR cDNA knock-in by homologous recombination. E, H, and B are restriction sites for _Eco_RI, _Hind_III, and _Bam_HI, respectively. Neo indicates the neomycin-resistance gene, and HSV-tk is the thymidine kinase gene under control of the herpes simplex virus promoter. The 5′-genomic probe used for DNA blot analysis is indicated by the hatched box. The positions of wild-type (pr 3) and mutant allele-specific (pr 2) primers and the common primer (pr 1) used in the genotyping PCR are indicated by arrowheads. The _Eco_RI restriction fragments detected with the 5′-genomic probe in the wild-type and targeted allele are denoted by horizontal bars. (B) DNA blot analyses of three recombinant ES clones. Genomic DNA was prepared from the ES clones (nos. 14, 25, and 58), and aliquots (10 μg) were digested by _Eco_RI. Eco_RI digestion generated 11.0- and 6.2-kb bands for the wild-type and targeted alleles, respectively, by using the 5′-genomic probe. (C) Genotyping of the Ahr gene by DNA blot analysis. Genomic DNA was extracted from the tails of heterozygous and homozygous h_AHR mice and wild-type _Ahr_b-1/b-1 mice and digested by Eco_RI for DNA blot analysis. (D) Genotyping of littermates from the intercrosses of heterozygotes. PCR fragments of wild-type amplified with pr1 and pr3 (Ahr_b-1; 280 bp) and mutant allele with pr1 and pr2 (h_AHR; 240 bp) as depicted in A. H/H, H/b, and b/b indicate homozygous and heterozygous h_AHR mice and wild type (_Ahr_b-1/b-1), respectively.
Figure 2
Expression of hAHR in multiple tissues of the hAHR knock-in mouse. (A) RNA blot analysis of polyA RNA (5 μg/lane) extracted from five representative organs of homozygous and heterozygous h_AHR_ mice and Ahr_b-1/b-1 mice. Human and mouse Ahr transcripts (hAHR and Ahrb-1, respectively) are indicated (Left). The same membrane was rehybridized with 32P-labeled cDNA of mouse GAPDH. H/H, H/b, and b/b are described in the Fig. 1 legend. (B) RT-PCR analyses of hAHR and murine Ahr mRNA expression in kidney and palate of GD18.5 fetuses. The reverse transcription was conducted either in the presence (+) or absence (−) of reverse transcriptase. PCR products representing the transcripts derived either from h_AHR or Ahr_b-1 are indicated on the left. (C and D) Immunohistochemical analysis of hAHR protein in the lung of a homozygous h_AHR mouse. Immunoreactivity of AHR protein was observed in the alveolar epithelial cells of homozygous h_AHR_ lung (C), whereas no immunoreactivity was observed in the lung of _Ahr_−/− mouse (D). Original magnifications, ×400 (C and D).
Figure 3
Inducible expression of AHR target genes. Northern blot analysis of AHR-regulated CYP1A1 and CYP1A2 was performed. Six-week-old homozygous h_AHR_, _Ahr_d/d, and _Ahr_b-1/b-1 mice were treated with 80 mg/kg 3-MC (A) or 100 μg/kg TCDD (B). Total hepatic RNA was isolated 24 h after treatment and subjected to Northern analysis (10 μg/lane). Equal loading was confirmed by the abundance of GAPDH transcripts.
Figure 4
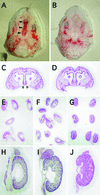
Fetal teratogenesis after maternal administration of TCDD. (A and C) Cleft palate in an Ahr_b-1/b-1 fetus is shown. Filled arrowheads in A and open arrowheads in C indicate the failure of palatine shelves to fuse. Note that homozygous h_AHR fetuses showed no cleft palates after TCDD treatment (B and D). (E, F, H, and I) Fetal hydronephrosis induced by TCDD. Ahr_b-1/b-1 (E and H) and homozygous h_AHR (F and I) fetuses are shown. (G and J) Unaffected kidneys from untreated _Ahr_b-1/b-1 fetuses are shown.
Similar articles
- The constitutively active Ah receptor (CA-Ahr) mouse as a potential model for dioxin exposure--effects in vital organs.
Brunnberg S, Andersson P, Lindstam M, Paulson I, Poellinger L, Hanberg A. Brunnberg S, et al. Toxicology. 2006 Jul 25;224(3):191-201. doi: 10.1016/j.tox.2006.04.045. Toxicology. 2006. PMID: 16766111 - Differential gene regulation by the human and mouse aryl hydrocarbon receptor.
Flaveny CA, Murray IA, Perdew GH. Flaveny CA, et al. Toxicol Sci. 2010 Apr;114(2):217-25. doi: 10.1093/toxsci/kfp308. Epub 2009 Dec 31. Toxicol Sci. 2010. PMID: 20044593 Free PMC article. - Aryl hydrocarbon receptor activation in genital tubercle, palate, and other embryonic tissues in 2,3,7, 8-tetrachlorodibenzo-p-dioxin-responsive lacZ mice.
Willey JJ, Stripp BR, Baggs RB, Gasiewicz TA. Willey JJ, et al. Toxicol Appl Pharmacol. 1998 Jul;151(1):33-44. doi: 10.1006/taap.1998.8444. Toxicol Appl Pharmacol. 1998. PMID: 9705885 - Ah receptor: dioxin-mediated toxic responses as hints to deregulated physiologic functions.
Bock KW, Köhle C. Bock KW, et al. Biochem Pharmacol. 2006 Aug 14;72(4):393-404. doi: 10.1016/j.bcp.2006.01.017. Epub 2006 Mar 20. Biochem Pharmacol. 2006. PMID: 16545780 Review. - [Exposure to dioxins in the environment and their health risk].
Tohyama C. Tohyama C. Nihon Eiseigaku Zasshi. 2006 Jan;61(1):5-10. doi: 10.1265/jjh.61.5. Nihon Eiseigaku Zasshi. 2006. PMID: 16506649 Review. Japanese.
Cited by
- The Role of Endocrine Disruption Chemical-Regulated Aryl Hydrocarbon Receptor Activity in the Pathogenesis of Pancreatic Diseases and Cancer.
Kim K. Kim K. Int J Mol Sci. 2024 Mar 29;25(7):3818. doi: 10.3390/ijms25073818. Int J Mol Sci. 2024. PMID: 38612627 Free PMC article. Review. - Evolutive emergence and divergence of an Ig regulatory node: An environmental sensor getting cues from the aryl hydrocarbon receptor?
D'Addabbo P, Frezza D, Sulentic CEW. D'Addabbo P, et al. Front Immunol. 2023 Feb 3;14:996119. doi: 10.3389/fimmu.2023.996119. eCollection 2023. Front Immunol. 2023. PMID: 36817426 Free PMC article. Review. - The Relationship between Typical Environmental Endocrine Disruptors and Kidney Disease.
Zhang X, Flaws JA, Spinella MJ, Irudayaraj J. Zhang X, et al. Toxics. 2022 Dec 29;11(1):32. doi: 10.3390/toxics11010032. Toxics. 2022. PMID: 36668758 Free PMC article. Review. - Characterization of marine-derived halogenated indoles as ligands of the aryl hydrocarbon receptor.
King J, Woolner VH, Keyzers RA, Rosengren RJ. King J, et al. Toxicol Rep. 2022 May 25;9:1198-1203. doi: 10.1016/j.toxrep.2022.05.016. eCollection 2022. Toxicol Rep. 2022. PMID: 36518459 Free PMC article. - Preclinical models of idiosyncratic drug-induced liver injury (iDILI): Moving towards prediction.
Segovia-Zafra A, Di Zeo-Sánchez DE, López-Gómez C, Pérez-Valdés Z, García-Fuentes E, Andrade RJ, Lucena MI, Villanueva-Paz M. Segovia-Zafra A, et al. Acta Pharm Sin B. 2021 Dec;11(12):3685-3726. doi: 10.1016/j.apsb.2021.11.013. Epub 2021 Nov 18. Acta Pharm Sin B. 2021. PMID: 35024301 Free PMC article. Review.
References
- Poland A, Knutson C. Annu Rev Pharmacol Toxicol. 1982;22:517–554. - PubMed
- Whitelock J P., Jr Annu Rev Pharmacol Toxicol. 1990;30:251–277. - PubMed
- Swanson H J, Bradfield C A. Pharmacogenetics. 1993;3:213–230. - PubMed
- Hoffman E C, Reyes H, Chu F-F, Sander F, Conly L H, Brooks B A, Hankinsin O. Science. 1991;252:954–958. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases