Deficiencies of GM-CSF and interferon gamma link inflammation and cancer - PubMed (original) (raw)
Deficiencies of GM-CSF and interferon gamma link inflammation and cancer
Thomas Enzler et al. J Exp Med. 2003.
Abstract
Chronic inflammation contributes to carcinogenesis, but the underlying mechanisms are poorly understood. We report that aged granulocyte-macrophage colony stimulating factor (GM-CSF)-deficient mice develop a systemic lupus erythematosis (SLE)-like disorder associated with the impaired phagocytosis of apoptotic cells. Concurrent deficiency of interferon (IFN)-gamma attenuates the SLE, but promotes the formation of diverse hematologic and solid neoplasms within a background of persistent infection and inflammation. Whereas activated B cells show a resistance to fas-induced apoptosis, antimicrobial therapy prevents lymphomagenesis and solid tumor development. These findings demonstrate that the interplay of infectious agents with cytokine-mediated regulation of immune homeostasis is a critical determinant of cancer susceptibility.
Figures
Figure 1.
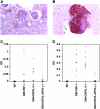
Aged GM-CSF– and GM-CSF/IL-3–deficient mice develop a SLE-like disorder. (A) Membrano-proliferative glomerulonephritis with Ig deposition in a GM-CSF-deficient mouse, anti-κ antibody, original magnification ×400. (B) B220+ B cell aggregates in the renal pelvis of a GM-CSF–deficient mouse, original magnification ×400. (C) Anti-double stranded DNA antibodies. (D) Anti-C1q reactivity.
Figure 2.
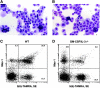
Impaired phagocytosis of apoptotic cells in GM-CSF– and GM-CSF/IL-3–deficient mice. Dexamethasone-induced apoptotic thymocytes from GM-CSF–deficient mice were injected into the peritoneal cavities of wild-type or GM-CSF/IL-3–deficient mice. Stained cytospins of harvested cells from: (A) wild-type mouse, original magnification ×400. (B) GM-CSF/IL-3–deficient mouse, original magnification ×400. Similar results were obtained in five experiments, and GM-CSF-deficient mice manifested comparable defects. (C and D) Apoptotic thymocytes were labeled with 5[6]-TAMRA before injection, and FACS® was used to analyze the harvested peritoneal cells. (C) Wild-type mouse. (D) GM-CSF/IL-3–deficient mouse. Similar results were found in six experiments.
Figure 3.
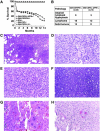
Infection, inflammation, and cancer in GM-CSF/IFN-γ– and GM-CSF/IL-3/IFN-γ–deficient mice. (A) Survival curves for mice singly or multiply deficient in GM-CSF, IL-3, and IFN-γ. The numbers of animals per cohort were: GM-CSF/IL-3/IFN-γ– (n = 29), GM-CSF/IFN-γ– (n = 44), IFN-γ– (n = 14), GM-CSF– (n = 18), GM-CSF/IL-3– (n = 18). (B) Spectrum and incidence of spontaneous tumors. The number of mice harboring tumors at autopsy is shown. (C) Lethal bacterial pneumonia, H&E stain, original magnification ×400. (D) Granulomatous inflammation with multinucleated giant cells, H&E stain, original magnification ×400. (E) Plasmacytoid lymphoma, H&E stain, original magnification ×400. (F) High grade large cell lymphoma, H&E stain, original magnification ×400. (G) Choriocarcinoma, H&E stain, original magnification ×200. (H) Mucin positive biliary tract/liver carcinoma, mucicarmine stain, original magnification ×400.
Figure 4.
B cell lymphomas. (A) Tumor-derived DNA from six different lymphomas was analyzed by southern using a Jκ probe. The germline band is 2.7 kb. (B) Single cell lymphoma suspensions were stimulated with anti-CD40 antibodies, and spectral karyotyping of metaphase spreads was performed. Shown are clonal 12:1, 4:13, 13:4, and 1:6 chromosomal translocations in one case. Chromosomal abnormalities were found in 3 of 6 tumors. (C) Impaired fas-mediated apoptosis. Enriched splenic B cells (90% pure) were stimulated for 48 h with anti-CD40 antibody (after activation, >90% B cells), and then 106 cell aliquots were incubated with 1 μg of anti-fas antibody, 100 μM etoposide, 1 μM staurosporine, or 2 μg/ml of actinomycin D for 12 h and cell viability determined. Data are pooled from six experiments; fas-treated wild-type versus fas-treated GM-CSF/IL-3/IFN-γ deficient, P < 0.0001. Similar results were obtained with LPS (50 μg) stimulated B cells. A comparable fas-resistance was also found with GM-CSF/IFN-γ–deficient B cells. (D) TNF-α and IFN-γ are required for fas-mediated B cell apoptosis. The data are pooled from four experiments.
Similar articles
- IFN-gamma limits macrophage expansion in MRL-Fas(lpr) autoimmune interstitial nephritis: a negative regulatory pathway.
Schwarting A, Moore K, Wada T, Tesch G, Yoon HJ, Kelley VR. Schwarting A, et al. J Immunol. 1998 Apr 15;160(8):4074-81. J Immunol. 1998. PMID: 9558118 - MFG-E8-mediated uptake of apoptotic cells by APCs links the pro- and antiinflammatory activities of GM-CSF.
Jinushi M, Nakazaki Y, Dougan M, Carrasco DR, Mihm M, Dranoff G. Jinushi M, et al. J Clin Invest. 2007 Jul;117(7):1902-13. doi: 10.1172/JCI30966. J Clin Invest. 2007. PMID: 17557120 Free PMC article. - Interferon-gamma enhances human eosinophil effector functions induced by granulocyte-macrophage colony-stimulating factor or interleukin-5.
Yamaguchi T, Kimura H, Kurabayashi M, Kozawa K, Kato M. Yamaguchi T, et al. Immunol Lett. 2008 Jun 15;118(1):88-95. doi: 10.1016/j.imlet.2008.03.005. Epub 2008 Apr 10. Immunol Lett. 2008. PMID: 18440651
Cited by
- Blood Growth Factor Levels in Patients with Systemic Lupus Erythematosus: High Neuregulin-1 Is Associated with Comorbid Cardiovascular Pathology.
Ermakov EA, Melamud MM, Boiko AS, Ivanova SA, Sizikov AE, Nevinsky GA, Buneva VN. Ermakov EA, et al. Life (Basel). 2024 Oct 14;14(10):1305. doi: 10.3390/life14101305. Life (Basel). 2024. PMID: 39459605 Free PMC article. - Long-term exposure to "low-dose" bisphenol A decreases mitochondrial DNA copy number, and accelerates telomere shortening in human CD8 + T cells.
Tran HTT, Herz C, Lamy E. Tran HTT, et al. Sci Rep. 2020 Sep 25;10(1):15786. doi: 10.1038/s41598-020-72546-x. Sci Rep. 2020. PMID: 32978426 Free PMC article. - Bacteria, inflammation and cancer.
Pollard J. Pollard J. Nat Rev Immunol. 2015 Sep 15;15(9):528. doi: 10.1038/nri3899. Epub 2015 Aug 14. Nat Rev Immunol. 2015. PMID: 26272296 No abstract available. - Glycolysis-Related Gene Signature Can Predict Survival and Immune Status of Hepatocellular Carcinoma.
Xu Q, Miao D, Song X, Chen Z, Zeng L, Zhao L, Xu J, Lin Z, Yu F. Xu Q, et al. Ann Surg Oncol. 2022 Jun;29(6):3963-3976. doi: 10.1245/s10434-022-11502-7. Epub 2022 Mar 9. Ann Surg Oncol. 2022. PMID: 35266081
References
- Mackay, I.R., and N.R. Rose. 2001. Autoimmunity and lymphoma: tribulations of B cells. Nat. Immunol. 2:793–795. - PubMed
- Kuper, H., H.O. Adami, and D. Trichopoulos. 2000. Infections as a major preventable cause of human cancer. J. Intern. Med. 248:171–183. - PubMed
- Ekbom, A., C. Helmick, M. Zack, and H.O. Adami. 1990. Ulcerative colitis and colorectal cancer. A population-based study. N. Engl. J. Med. 323:1228–1233. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous