Mesenchymal progenitor self-renewal deficiency leads to age-dependent osteoporosis in Sca-1/Ly-6A null mice - PubMed (original) (raw)
Mesenchymal progenitor self-renewal deficiency leads to age-dependent osteoporosis in Sca-1/Ly-6A null mice
Mortaza Bonyadi et al. Proc Natl Acad Sci U S A. 2003.
Abstract
The cellular and molecular mechanisms that underlie age-dependent osteoporosis, the most common disease in the Western Hemisphere, are poorly understood in part due to the lack of appropriate animal models in which to study disease progression. Here, we present a model that shows many similarities to the human disease. Sca-1, well known for its expression on hematopoietic stem cells, is present on a subset of bone marrow stromal cells, which potentially include mesenchymal stem cells. Longitudinal studies showed that Sca-1(-/-) mice undergo normal bone development but with age exhibit dramatically decreased bone mass resulting in brittle bones. In vivo and in vitro analyses demonstrated that Sca-1 is required directly for the self-renewal of mesenchymal progenitors and indirectly for the regulation of osteoclast differentiation. Thus, defective mesenchymal stem or progenitor cell self-renewal may represent a previously uncharacterized mechanism of age-dependent osteoporosis in humans.
Figures
Figure 1
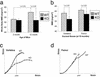
BMD and mechanical analysis of _Sca-1_−/− (KO) and Sca-1+/+ mice (WT) demonstrate that _Sca-1_−/− mice exhibit age-related osteoporosis. (a) Significantly higher (P < 0.0001) whole-body BMD was observed in control mice at 12 months of age compared with _Sca-1_−/− animals, whereas no significant differences were observed at 2 months of age. (b) The BMD of excised femurs and L6 vertebrae at 15 months shows significantly less mineralization in _Sca-1_−/− femurs and vertebrae than those of WT mice, with a greater difference in the vertebrae. (c) Vertebral stress-strain response at 15 months demonstrated that vertebrae from KO were substantially weaker and more compliant than bones from WT mice. (d) Femoral stress–strain response at 15 months showed that femurs from KO were more brittle and weak than femurs from controls, although equally as stiff.
Figure 2
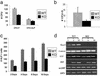
Sca-1−/− mice have a non-cell autonomous osteoclast defect. (a) The absolute number of TRAP+ (osteoclasts) cells in femurs of both 2- and 9-month-old _Sca-1_−/− mice is <40% of that in Sca-1+/+ mice. (b) Osteoblast (OB)/splenocytes (monocyte, MO) coculture experiments. Sca-1+/+ (WT) osteoblast (2 × 104 cells per well) and splenic (1.5 × 105 cells per well) cultures were used to standardize the culture results (i.e., 100% on x axis); osteoclast formation in all other culture combinations is specified as the percentage compared with the WT cocultures. The number of TRAP+ cells in cocultures in which monocytes are derived from WT mice and osteoblasts are derived from either _Sca-1_−/− or WT mice is significantly different (P < 0.001). However, osteoclast formation is not significantly altered in cocultures in which OB are derived from KO and monocytes from either _Sca-1_−/− or WT mice (P = 0.4). (c) The number of mononucleated, binucleated, and multinucleated osteoclasts, and total number of osteoclasts (TRAP+ cells) generated in vitro by different combinations of osteoblasts and monocytes derived from WT or _Sca-1_−/− mice. (d) Images of TRAP+ mononucleated, binucleated, and multinucleated cells generated by a combination of monocytes and osteoblasts derived from _Sca-1_−/− mice in culture.
Figure 3
Progressive diminution of bone mass in _Sca-1_−/− mice. (a) Plastic sections of femurs from _Sca-1_−/− and Sca-1+/+ mice at 4 and 15 months of age used for image analysis. (b) Image analysis demonstrates that _Sca-1_−/− femurs have 27% (P < 0.001) fewer trabeculae compared with control bones at 4 months of age and 58% (P < 0.001) compared with Sca-1+/+ bones at 15 months of age. (c) Goldner's trichrome staining of unmineralized bone sections of femur showing mineralized bone (green), osteoid (red), and cartilage (pink) was used to measure osteoid volume and osteoid surface. (d and e) The percent osteoid volume and osteoid surface calculated from unmineralized femoral sections from 4- and 15-month-old _Sca-1_−/− mice are less than those of Sca-1+/+ mice. (f) Double calcein labeling of bones from _Sca-1_−/− and Sca-1+/+ mice at 2 and 6 months of age revealed that bone formation in _Sca-1_−/− mice is essentially normal at 2 months of age but is dramatically reduced by 6 months of age.
Figure 4
In vitro analysis of the cellular and molecular basis of the bone formation deficiency in _Sca-1_−/− mice. (a) The numbers of CFU-F and CFU-ALP generated in BM cultures derived from 2-month-old _Sca-1_−/− mice are <50% of those derived from Sca-1+/+ mice. (b) The number of CFU-O formed from _Sca-1_−/− cultures is ≈50% of CFU-O from Sca-1+/+ cultures. (c) The number of ALP+ cells in calvarial cultures from _Sca-1_−/− at different time points is consistently less than that of Sca-1+/+ mice. The cultures were initiated with 10,000 cells. (d) RT-PCR analysis of osteoblast- and adipocyte-associated genes during in vitro differentiation at 3, 6, and 10 days excludes the possible disturbances of osteoblast and/or adipocyte pathways in _Sca-1_−/− mice. Expression of adipsin (mADP), alkaline phosphatase (ALP), osteopontin (OPN), Sca-1, and bone sialoprotein (BSP) genes were examined, and L32 expression was used as a control for relative mRNA levels.
Figure 5
Mesenchymal progenitor self-renewal deficiency in _Sca-1_−/− mice. (a) Total number of CFU-F formed in _Sca-1_−/− compared with Sca-1+/+ cultures is reduced 45% and 67%, respectively, in 2- and 7-month-old mice. (b) The total numbers of CFU-F and CFU-ALP are decreased in primary BM stromal cultures derived from 2- and 7-month-old _Sca-1_−/− cultures; however, the ratio of CFU-ALP/CFU-F is consistent (≈10%) between KO and WT cultures, indicating that the decrease in osteoprogenitors is due to a reduced total number of stroma progenitors in _Sca-1_−/− mice. (c) Total number of CFU-F in secondary BM stromal cultures derived from 2- and 7-month-old _Sca-1_−/− mice is ≈50% of those derived from Sca-1+/+ mice, suggesting a self-renewal deficiency by _Sca-1_−/− stromal progenitors. (d) Reduced CFU-A (adipocyte) was generated in BM stromal cultures derived from _Sca-1_−/− mice.
Similar articles
- Longitudinal analysis of mesenchymal progenitors and bone quality in the stem cell antigen-1-null osteoporotic mouse.
Holmes C, Khan TS, Owen C, Ciliberti N, Grynpas MD, Stanford WL. Holmes C, et al. J Bone Miner Res. 2007 Sep;22(9):1373-86. doi: 10.1359/jbmr.070604. J Bone Miner Res. 2007. PMID: 17547535 - Hematopoietic stem cell and progenitor defects in Sca-1/Ly-6A-null mice.
Ito CY, Li CY, Bernstein A, Dick JE, Stanford WL. Ito CY, et al. Blood. 2003 Jan 15;101(2):517-23. doi: 10.1182/blood-2002-06-1918. Epub 2002 Aug 29. Blood. 2003. PMID: 12393491 - Roles of Sca-1 in hematopoietic stem/progenitor cell function.
Bradfute SB, Graubert TA, Goodell MA. Bradfute SB, et al. Exp Hematol. 2005 Jul;33(7):836-43. doi: 10.1016/j.exphem.2005.04.001. Exp Hematol. 2005. PMID: 15963860 - Bone-Specific Overexpression of PITX1 Induces Senile Osteoporosis in Mice Through Deficient Self-Renewal of Mesenchymal Progenitors and Wnt Pathway Inhibition.
Karam N, Lavoie JF, St-Jacques B, Bouhanik S, Franco A, Ladoul N, Moreau A. Karam N, et al. Sci Rep. 2019 Mar 5;9(1):3544. doi: 10.1038/s41598-019-40274-6. Sci Rep. 2019. PMID: 30837642 Free PMC article. - Concise review: stem cell antigen-1: expression, function, and enigma.
Holmes C, Stanford WL. Holmes C, et al. Stem Cells. 2007 Jun;25(6):1339-47. doi: 10.1634/stemcells.2006-0644. Epub 2007 Mar 22. Stem Cells. 2007. PMID: 17379763 Review.
Cited by
- Isolation of multilineage progenitors from mouse brain.
Chen CC, Huang HI, Louis CL, Lin KT, Ron Y. Chen CC, et al. In Vitro Cell Dev Biol Anim. 2013 May;49(5):307-14. doi: 10.1007/s11626-013-9625-1. Epub 2013 May 1. In Vitro Cell Dev Biol Anim. 2013. PMID: 23636940 - Mesenchymal Stem Cell-Derived Extracellular Vesicle: A Promising Alternative Therapy for Osteoporosis.
Lu CH, Chen YA, Ke CC, Liu RS. Lu CH, et al. Int J Mol Sci. 2021 Nov 25;22(23):12750. doi: 10.3390/ijms222312750. Int J Mol Sci. 2021. PMID: 34884554 Free PMC article. Review. - Diet-induced obesity in stem cell antigen-1 KO mice.
Staszkiewicz J, Gimble JM, Dietrich MA, Gawronska-Kozak B. Staszkiewicz J, et al. Stem Cells Dev. 2012 Jan 20;21(2):249-59. doi: 10.1089/scd.2010.0507. Epub 2011 Jun 1. Stem Cells Dev. 2012. PMID: 21510817 Free PMC article. - Local delivery of tetramethylpyrazine eliminates the senescent phenotype of bone marrow mesenchymal stromal cells and creates an anti-inflammatory and angiogenic environment in aging mice.
Gao B, Lin X, Jing H, Fan J, Ji C, Jie Q, Zheng C, Wang D, Xu X, Hu Y, Lu W, Luo Z, Yang L. Gao B, et al. Aging Cell. 2018 Jun;17(3):e12741. doi: 10.1111/acel.12741. Epub 2018 Feb 28. Aging Cell. 2018. PMID: 29488314 Free PMC article. - Concise Review: Musculoskeletal Stem Cells to Treat Age-Related Osteoporosis.
Kiernan J, Davies JE, Stanford WL. Kiernan J, et al. Stem Cells Transl Med. 2017 Oct;6(10):1930-1939. doi: 10.1002/sctm.17-0054. Epub 2017 Aug 18. Stem Cells Transl Med. 2017. PMID: 28834263 Free PMC article. Review.
References
- Wasnich R D. In: Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Favus M J, editor. Philadelphia: Lippincott Williams & Wilkins; 1996. pp. 249–251.
- Bellows C G, Wang Y H, Heersche J N M, Aubin J E. Endocrinology. 1994;134:2221–2229. - PubMed
- Aubin J E. J Cell Biochem. 1998;Suppl. 30/31:73–82. - PubMed
- Roodman G D. Exp Hematol. 1999;27:1229–1241. - PubMed
- Spangrude G J, Heimfeld S, Weissman I L. Science. 1988;241:58–62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials