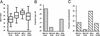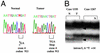Prevalence of defective DNA mismatch repair and MSH6 mutation in an unselected series of endometrial cancers - PubMed (original) (raw)
Prevalence of defective DNA mismatch repair and MSH6 mutation in an unselected series of endometrial cancers
Paul J Goodfellow et al. Proc Natl Acad Sci U S A. 2003.
Abstract
Endometrial cancer is the most common gynecologic malignancy in the United States and the most frequent extracolonic tumor in hereditary nonpolyposis colorectal cancer (HNPCC). HNPCC patients have inherited defects in DNA mismatch repair and the microsatellite instability (MSI) tumor phenotype. Sporadic endometrial cancers also exhibit MSI, usually associated with methylation of the MLH1 promoter. Germ-line MSH6 mutations, which are rare in HNPCC, have been reported in several families with multiple members affected with endometrial carcinoma. We reasoned that MSH6 mutation might account for loss of mismatch repair in MSI-positive endometrial cancers in which the cause of MSI is unknown. We therefore investigated MSI and MLH1 promoter methylation in 441 endometrial cancer patients unselected for age or personal and family history of cancers. MSI and MLH1 promoter methylation status were associated with age of onset and tumor histology. One hundred cases (23% of the entire series) were evaluated for MSH6 defects. Inactivating germ-line MSH6 mutations were identified in seven women with MSI-positive, MLH1 promoter unmethylated cancers. Most of the MSI in these cases was seen with mononucleotide repeat markers. The MSH6 mutation carriers were significantly younger than the rest of the population (mean age 54.8 versus 64.6, P = 0.04). Somatic mutations were seen in 17 tumors, all of which had MSI. Our data suggest that inherited defects in MSH6 in women with endometrial cancer are relatively common. The minimum estimate of the prevalence of inherited MSH6 mutation in endometrial cancer is 1.6% (7 of 441), comparable with the predicted prevalence for patients with colorectal cancer.
Figures
Figure 1
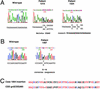
Flow diagram for results of testing for defects in DNA mismatch repair in 441 unselected patients with endometrial cancer. All tumors were assessed for MSI, and those with MSI were evaluated for MLH1 promoter methylation. Among the 35 cases lacking MLH1 methylation, five were previously shown to have germ-line MSH2 mutations (ref. and our unpublished data). The remaining 30 cases, classified as MSI-H unmethylated, were assessed for MSH6 mutations along with 10 MSI-L, 30 MSI-H methylated, and 30 MSS cases.
Figure 2
Association between MSI status and MLH1 promoter methylation and age at diagnosis and tumor type. (A) Box plot showing the distribution of ages among women with MSI-H unmethylated, MSI-H methylated, MSI-L, and MSS tumors. Boxes include the 25th through 75th centile for each group, and the median for each is shown with a horizontal bar. (B) Proportions of each group with onset of endometrial cancer before age 50. (C) Proportion of each group with nonendometrioid tumors (papillary serous, clear cell, or mixed histologies). MSI-H U, MSI-H MLH1 promoter unmethylated; MSI-H M, MSI-H MLH1 promoter methylated.
Figure 3
Germ-line mutations in endometrial cancer patients. (A) Representative DNA sequence analyses reveal mutations in exon 9. The overlapping sequences reflect a deletion in patient 1319 and an insertion in patient 1524. (B) Single base C → T substitution at codon 911 in patient 1497. Reverse sequence is shown (G → A). (C) Alignment of MSH6 peptide sequence with the Ala insertion in case 1064 within the ATPase domain of DNA mismatch repair MUTS family. Conserved amino acids are shown in red. The Ala insertion (A), marked with an asterisk, disrupts at the conserved VPAE sequence.
Figure 4
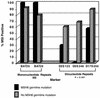
Reduced numbers of MSI events involving dinucleotide repeats in tumors from women with inherited MSH6 mutations. The rates of MSI at each of the five markers tested are compared for MSH6 mutation carriers (n = 7) and the 53 other cases classified as MSI-H. The combined number of MSI events seen with dinucleotide repeat markers (D2S123, D5S346, and D17S250) is significantly less in MSH6 mutation carriers than in the other MSI-H cases (P < 0.001, Fisher's exact test). NS, not significant.
Figure 5
Somatic MSH6 deletion in tumor 1335. (A) Germ-line C → T mutation at codon 911 (Arg911Stop) is heterozygous in the normal cellular DNA. (B) Allelic deletion in tumor 1335. Patient 1335 is heterozygous for the intron 5 variant detected by SSCV. Arrowheads indicate bands that reveal loss of heterozygosity.
Similar articles
- MSI in endometrial carcinoma: absence of MLH1 promoter methylation is associated with increased familial risk for cancers.
Whelan AJ, Babb S, Mutch DG, Rader J, Herzog TJ, Todd C, Ivanovich JL, Goodfellow PJ. Whelan AJ, et al. Int J Cancer. 2002 Jun 10;99(5):697-704. doi: 10.1002/ijc.10429. Int J Cancer. 2002. PMID: 12115503 - Prediction of a mismatch repair gene defect by microsatellite instability and immunohistochemical analysis in endometrial tumours from HNPCC patients.
de Leeuw WJ, Dierssen J, Vasen HF, Wijnen JT, Kenter GG, Meijers-Heijboer H, Brocker-Vriends A, Stormorken A, Moller P, Menko F, Cornelisse CJ, Morreau H. de Leeuw WJ, et al. J Pathol. 2000 Nov;192(3):328-35. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH701>3.0.CO;2-2. J Pathol. 2000. PMID: 11054716 - Increased risk for hereditary nonpolyposis colorectal cancer-associated synchronous and metachronous malignancies in patients with microsatellite instability-positive endometrial carcinoma lacking MLH1 promoter methylation.
Buttin BM, Powell MA, Mutch DG, Rader JS, Herzog TJ, Gibb RK, Huettner P, Edmonston TB, Goodfellow PJ. Buttin BM, et al. Clin Cancer Res. 2004 Jan 15;10(2):481-90. doi: 10.1158/1078-0432.ccr-1110-03. Clin Cancer Res. 2004. PMID: 14760069 - Hereditary nonpolyposis colorectal cancer: diagnostic strategies and their implications.
Bonis PA, Trikalinos TA, Chung M, Chew P, Ip S, DeVine DA, Lau J. Bonis PA, et al. Evid Rep Technol Assess (Full Rep). 2007 May;(150):1-180. Evid Rep Technol Assess (Full Rep). 2007. PMID: 17764220 Free PMC article. Review. - Do MSH6 mutations contribute to double primary cancers of the colorectum and endometrium?
Charames GS, Millar AL, Pal T, Narod S, Bapat B. Charames GS, et al. Hum Genet. 2000 Dec;107(6):623-9. doi: 10.1007/s004390000417. Hum Genet. 2000. PMID: 11153917 Review.
Cited by
- Universal testing in endometrial cancer in Sweden.
Andersson E, Keränen A, Lagerstedt-Robinson K, Ghazi S, Lindblom A, Tham E, Mints M. Andersson E, et al. Hered Cancer Clin Pract. 2024 Aug 22;22(1):14. doi: 10.1186/s13053-024-00288-2. Hered Cancer Clin Pract. 2024. PMID: 39175077 Free PMC article. - Characterization of mismatch-repair/microsatellite instability-discordant endometrial cancers.
Riedinger CJ, Esnakula A, Haight PJ, Suarez AA, Chen W, Gillespie J, Villacres A, Chassen A, Cohn DE, Goodfellow PJ, Cosgrove CM. Riedinger CJ, et al. Cancer. 2024 Feb 1;130(3):385-399. doi: 10.1002/cncr.35030. Epub 2023 Sep 26. Cancer. 2024. PMID: 37751191 Review. - A retrospective study of consistency between immunohistochemistry and polymerase chain reaction of microsatellite instability in endometrial cancer.
Wang C, Kuang W, Zeng J, Ren Y, Liu Q, Sun H, Feng M, Liang D. Wang C, et al. PeerJ. 2023 Aug 28;11:e15920. doi: 10.7717/peerj.15920. eCollection 2023. PeerJ. 2023. PMID: 37663290 Free PMC article. - Japanese Society of Medical Oncology/Japan Society of Clinical Oncology/Japanese Society of Pediatric Hematology/Oncology-led clinical recommendations on the diagnosis and use of immunotherapy in patients with DNA mismatch repair deficient (dMMR) tumors, third edition.
Mishima S, Naito Y, Akagi K, Hayashi N, Hirasawa A, Hishiki T, Igarashi A, Ikeda M, Kadowaki S, Kajiyama H, Kato M, Kenmotsu H, Kodera Y, Komine K, Koyama T, Maeda O, Miyachi M, Nishihara H, Nishiyama H, Ohga S, Okamoto W, Oki E, Ono S, Sanada M, Sekine I, Takano T, Tao K, Terashima K, Tsuchihara K, Yatabe Y, Yoshino T, Baba E. Mishima S, et al. Int J Clin Oncol. 2023 Oct;28(10):1237-1258. doi: 10.1007/s10147-023-02397-9. Epub 2023 Aug 20. Int J Clin Oncol. 2023. PMID: 37599324 Free PMC article. - Alternative splicing of PSMD13 mediated by genetic variants is significantly associated with endometrial cancer risk.
He S, Cao R, Mao Y, Li N, Wang Y, Ma H, Tian K. He S, et al. J Gynecol Oncol. 2023 May;34(3):e40. doi: 10.3802/jgo.2023.34.e40. Epub 2023 Jan 23. J Gynecol Oncol. 2023. PMID: 36731897 Free PMC article.
References
- Wijnen J, De Leeuw W, Vasen H, van der Klift H, Møller P, Stormorken A. Nat Genet. 1999;23:142–144. - PubMed
- Miyaki M, Konishi M, Tanaka K, Kikuchi-Yanoshita R, Muraoka M, Yasuno M, Igari T, Koike M, Chiba M, Mori T. Nat Genet. 1997;17:271–272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA071754/CA/NCI NIH HHS/United States
- U10 CA27469/CA/NCI NIH HHS/United States
- U10 CA027469/CA/NCI NIH HHS/United States
- P30 CA91842/CA/NCI NIH HHS/United States
- P30 CA091842/CA/NCI NIH HHS/United States
- CA71754/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous