Postinsult treatment with lithium reduces brain damage and facilitates neurological recovery in a rat ischemia/reperfusion model - PubMed (original) (raw)
Postinsult treatment with lithium reduces brain damage and facilitates neurological recovery in a rat ischemia/reperfusion model
Ming Ren et al. Proc Natl Acad Sci U S A. 2003.
Abstract
Lithium has long been a primary drug used to treat bipolar mood disorder, even though the drug's therapeutic mechanisms remain obscure. Recent studies demonstrate that lithium has neuroprotective effects against glutamate-induced excitotoxicity in cultured neurons and in vivo. The present study was undertaken to examine whether postinsult treatment with lithium reduces brain damage induced by cerebral ischemia. We found that s.c. injection of lithium dose dependently (0.5-3 mEq/kg) reduced infarct volume in the rat model of middle cerebral artery occlusionreperfusion. Infarct volume was reduced at a therapeutic dose of 1 mEq/kg even when administered up to 3 h after the onset of ischemia. Neurological deficits induced by ischemia were also reduced by daily administration of lithium over 1 week. Moreover, lithium treatment decreased the number of neurons showing DNA damage in the ischemic brain. These neuroprotective effects were associated with an up-regulation of cytoprotective heat shock protein 70 (HSP70) in the ischemic brain hemisphere as determined by immunohistochemistry and Western blotting analysis. Lithium-induced HSP70 up-regulation in the ischemic hemisphere was preceded by an increase in the DNA binding activity of heat shock factor 1, which regulates the transcription of HSP70. Physical variables and cerebral blood flow were unchanged by lithium treatment. Our results suggest that postinsult lithium treatment reduces both ischemia-induced brain damage and associated neurological deficits. Moreover, the heat shock response is likely to be involved in lithium's neuroprotective actions. Additionally, our studies indicate that lithium may have clinical utility for the treatment of patients with acute stroke.
Figures
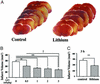
Figure 1
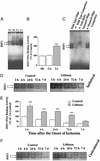
Postinsult lithium treatment reduces MCAO/reperfusion-induced brain infarction. Rats were s.c.-injected with normal saline or LiCl after MCAO and killed at different time points, and the brains were stained with 2,3,5-triphenyltetrazolium chloride. (A) The animals were subjected to ischemia for 1 h followed by reperfusion for 23 h and then killed for the determination of infarct volume. LiCl (3 mEq/kg) was injected immediately after cerebral ischemia induction. (B) A single injection of the indicated dose of LiCl was performed in rats immediately after the onset of MCAO and the animals were killed 24 h later. (C) LiCl (1 mEq/kg) was injected at 3 h after the onset of MCAO, and the animals were killed at 24 h after the onset of MCAO. Data are means ± SEM from eight rats in each group. **, P < 0.01; ***, P < 0.001 compared with the control (saline-injected group). †, P < 0.05, between the groups of 0.5 mEq/kg and 3.0 mEq/kg shown in B.
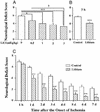
Figure 2
Lithium treatment reduces MCAO/reperfusion-induced neurological deficits. (A) Rats were subjected to MCAO for 1 h followed by reperfusion for 23 h before determination of neurological deficits. When used, indicated doses of LiCl were injected immediately after MCAO. (B) Rats were injected with LiCl (1 mEq/kg) 3 h after the onset of MCAO and evaluated for neurological deficits 24 h postinsult. (C) The animals were injected with LiCl (1 mEq/kg) or normal saline immediately after MCAO and followed by daily injections for up to 6 days. Neurological deficits were evaluated once a day throughout a 7-day postischemic period. Data are means ± SEM from eight rats in each group. *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared with the corresponding saline-control group; †, P < 0.05 between the groups of 0.5 mEq/kg and 3.0 mEq/kg shown in A.
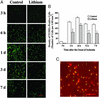
Figure 3
Lithium suppresses MCAO/reperfusion-induced DNA damage in rat brain. Rats were subjected to MCAO for 1 h followed by reperfusion for the indicated times. LiCl (1 mEq/kg) or normal saline was injected immediately after MCAO and daily thereafter as indicated. Brains were sliced with a cryostat into sections of 10-μm thickness and evaluated by TUNEL assay. The sections were also double-stained with NeuN. (A) Representative time-dependent changes in TUNEL staining in a defined locus within the cortical ischemic penumbra area of saline- and lithium-treated rats. (B) Quantification of the density of TUNEL-positive cells shown in A. (C) At 72 h after the ischemic insult, a great majority of TUNEL-positive cells (green) were colabeled with NeuN (red) in the ischemic penumbra area. Bar graphs shown are means ± SEM of TUNEL-positive cells from four rats in each group. *, P < 0.05; **, P < 0.01, compared with the corresponding saline-control group. (Bar: 100 μm.)
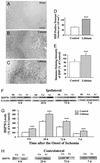
Figure 4
Lithium enhances MCAO/reperfusion-induced HSP70 levels. Rats were subjected to MCAO for 1 h followed by reperfusion until death at the indicated times. LiCl (1 mEq/kg) or saline was injected immediately after the onset of MCAO followed by daily injections. Representative immunohistochemistry for HSP70-positive cells within the penumbra of ischemic cerebral cortex or in the corresponding area of cortex was examined in sham-operated rats (A), saline-control rats (1 h of MCAO followed by 23 h of reperfusion) (B), and lithium-treated rats (1 h of MCAO followed by 23 h of reperfusion) (C). (D) The number of HSP70-positive cells from B and C were quantified in corresponding 1.05-mm2 areas. (E) The intensity of HSP70-positive cells from B and C were quantified by using National Institutes of Health IMAGE 1.61 software and the data are expressed as percentage of saline control. (F) Western blotting analysis of HSP70 protein levels in extracts from the entire ischemic cortex of the ipsilateral hemisphere of saline-treated control (Co) and lithium-treated (Li) rats and the corresponding area from sham-operated (Sh) rats. Levels of β-actin protein were used as the control. (G) Quantified results of Western blotting were expressed as means ± SEM of percentage of 6 h control from four rats in each group. ***, P < 0.001 compared with corresponding saline controls. (Bar: 200 μm.) (H) Western blotting analysis of HSP70 protein levels in extracts from the corresponding area in the cortex of the contralateral hemisphere of saline-treated control (Co) and lithium-treated (Li) rats.
Figure 5
Lithium increases HSF1 DNA binding activity in ischemic brain. (A) Rats were subjected to MCAO for 1 h followed by 2 h of reperfusion. LiCl (1 mEq/kg) or normal saline was injected immediately after MCAO. HSF1 DNA binding activities were determined by EMSA from extracts of the entire ischemic cortex of rats. The HSF–HSE complex migrated as a doublet in normal (No), sham-operated (Sh), saline control (Co), and lithium-treated (Li) groups as indicated by arrows. (B) Quantified EMSA results shown in A. (C) The specificity of HSF1 binding to HSE was assessed by the presence of 60-fold excess of unlabeled HSE, addition of 4 μl of rabbit anti-HSF1 antibody (200 μg/0.1 ml, Stressgen Bioreagents) to the EMSA mixture, the binding activity of 32P-labeled, mutated HSE (5′-CTA-TAA-TCT-TGT-ATA-AGT-TTG-TAG-3′), and WT HSE binding in the presence of 60-fold excess of unlabeled, mutated HSE. (D) Rats were subjected to MCAO for 1 h followed by reperfusion for the indicated times. LiCl (1 mEq/kg) or normal saline was injected immediately after MCAO followed by daily injections. The time course of lithium-induced enhancement of HSF1 DNA binding activity was determined by EMSA from extracts of entire ischemic cortex of saline-treated control and lithium-treated rats. (E) Quantified results of EMSA results shown in D. Data are means ± SEM of percentage of 3 h control from four rats in each group. ***, P < 0.001 compared with respective saline controls. (F) Time course of HSF1 DNA binding activity determined by EMSA in extracts of the corresponding areas in the contralateral hemisphere of saline-treated control and lithium-treated rats.
Similar articles
- Synergistic effects of prostaglandin E1 and lithium in a rat model of cerebral ischemia.
Han R, Gao B, Sheng R, Zhang LS, Zhang HL, Gu ZL, Qin ZH. Han R, et al. Acta Pharmacol Sin. 2008 Oct;29(10):1141-9. doi: 10.1111/j.1745-7254.2008.00873.x. Acta Pharmacol Sin. 2008. PMID: 18817617 - Enhancement of neuroprotection and heat shock protein induction by combined prostaglandin A1 and lithium in rodent models of focal ischemia.
Xu XH, Zhang HL, Han R, Gu ZL, Qin ZH. Xu XH, et al. Brain Res. 2006 Aug 2;1102(1):154-62. doi: 10.1016/j.brainres.2006.04.111. Epub 2006 Jun 22. Brain Res. 2006. PMID: 16797496 - [Neuroprotective actions of lithium].
Hashimoto R, Fujimaki K, Jeong MR, Senatorov VV, Christ L, Leeds P, Chuang DM, Takeda M. Hashimoto R, et al. Seishin Shinkeigaku Zasshi. 2003;105(1):81-6. Seishin Shinkeigaku Zasshi. 2003. PMID: 12701214 Review. Japanese. - The antiapoptotic actions of mood stabilizers: molecular mechanisms and therapeutic potentials.
Chuang DM. Chuang DM. Ann N Y Acad Sci. 2005 Aug;1053:195-204. doi: 10.1196/annals.1344.018. Ann N Y Acad Sci. 2005. PMID: 16179524 Review.
Cited by
- TRPM2 enhances ischemic excitotoxicity by associating with PKCγ.
Zong P, Feng J, Legere N, Li Y, Yue Z, Li CX, Mori Y, Miller B, Hao B, Yue L. Zong P, et al. Cell Rep. 2024 Feb 27;43(2):113722. doi: 10.1016/j.celrep.2024.113722. Epub 2024 Feb 2. Cell Rep. 2024. PMID: 38308841 Free PMC article. - In search of the Holy Grail for the treatment of neurodegenerative disorders: has a simple cation been overlooked?
Chuang DM, Manji HK. Chuang DM, et al. Biol Psychiatry. 2007 Jul 1;62(1):4-6. doi: 10.1016/j.biopsych.2007.04.008. Biol Psychiatry. 2007. PMID: 17572175 Free PMC article. Review. No abstract available. - Molecular actions and therapeutic potential of lithium in preclinical and clinical studies of CNS disorders.
Chiu CT, Chuang DM. Chiu CT, et al. Pharmacol Ther. 2010 Nov;128(2):281-304. doi: 10.1016/j.pharmthera.2010.07.006. Epub 2010 Aug 10. Pharmacol Ther. 2010. PMID: 20705090 Free PMC article. Review. - Lithium promotes DNA stability and survival of ischemic retinal neurocytes by upregulating DNA ligase IV.
Yang Y, Wu N, Tian S, Li F, Hu H, Chen P, Cai X, Xu L, Zhang J, Chen Z, Ge J, Yu K, Zhuang J. Yang Y, et al. Cell Death Dis. 2016 Nov 17;7(11):e2473. doi: 10.1038/cddis.2016.341. Cell Death Dis. 2016. PMID: 27853172 Free PMC article. - Lithium modulates miR-1906 levels of mesenchymal stem cell-derived extracellular vesicles contributing to poststroke neuroprotection by toll-like receptor 4 regulation.
Haupt M, Zheng X, Kuang Y, Lieschke S, Janssen L, Bosche B, Jin F, Hein K, Kilic E, Venkataramani V, Hermann DM, Bähr M, Doeppner TR. Haupt M, et al. Stem Cells Transl Med. 2021 Mar;10(3):357-373. doi: 10.1002/sctm.20-0086. Epub 2020 Nov 4. Stem Cells Transl Med. 2021. PMID: 33146943 Free PMC article.
References
- Manji H K, Moore G J, Chen G. Biol Psychiatry. 1999;46:929–940. - PubMed
- Grimes C A, Jope R S. Prog Neurobiol. 2001;65:391–426. - PubMed
- Chuang D-M, Chen R W, Chalecka-Franaszek E, Ren M, Hashimoto R, Senatorov V, Kanai H, Hough C, Hiroi T, Leeds P. Bipolar Disorders. 2002;4:1–9. - PubMed
- Hashimoto R, Hough C, Nakazawa T, Yamamoto T, Chuang D-M. J Neurochem. 2002;80:589–597. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical