Neural cell adhesion molecule (NCAM) association with PKCbeta2 via betaI spectrin is implicated in NCAM-mediated neurite outgrowth - PubMed (original) (raw)
Neural cell adhesion molecule (NCAM) association with PKCbeta2 via betaI spectrin is implicated in NCAM-mediated neurite outgrowth
Iryna Leshchyns'ka et al. J Cell Biol. 2003.
Abstract
In hippocampal neurons and transfected CHO cells, neural cell adhesion molecule (NCAM) 120, NCAM140, and NCAM180 form Triton X-100-insoluble complexes with betaI spectrin. Heteromeric spectrin (alphaIbetaI) binds to the intracellular domain of NCAM180, and isolated spectrin subunits bind to both NCAM180 and NCAM140, as does the betaI spectrin fragment encompassing second and third spectrin repeats (betaI2-3). In NCAM120-transfected cells, betaI spectrin is detectable predominantly in lipid rafts. Treatment of cells with methyl-beta-cyclodextrin disrupts the NCAM120-spectrin complex, implicating lipid rafts as a platform linking NCAM120 and spectrin. NCAM140/NCAM180-betaI spectrin complexes do not depend on raft integrity and are located both in rafts and raft-free membrane domains. PKCbeta2 forms detergent-insoluble complexes with NCAM140/NCAM180 and spectrin. Activation of NCAM enhances the formation of NCAM140/NCAM180-spectrin-PKCbeta2 complexes and results in their redistribution to lipid rafts. The complex is disrupted by the expression of dominant-negative betaI2-3, which impairs binding of spectrin to NCAM, implicating spectrin as the bridge between PKCbeta2 and NCAM140 or NCAM180. Redistribution of PKCbeta2 to NCAM-spectrin complexes is also blocked by a specific fibroblast growth factor receptor inhibitor. Furthermore, transfection with betaI2-3 inhibits NCAM-induced neurite outgrowth, showing that formation of the NCAM-spectrin-PKCbeta2 complex is necessary for NCAM-mediated neurite outgrowth.
Figures
Figure 1.
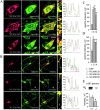
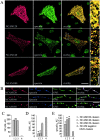
NCAM120, NCAM140, and NCAM180 colocalize with spectrin and increase its steady-state level. Double immunostaining of (A) CHO cells and (B) hippocampal neurons from NCAM−/− mice transfected with NCAM120, NCAM140, or NCAM180 with antibodies against NCAM and spectrin. Note the colocalization of all NCAM isoforms with spectrin. Density profiles of NCAM and spectrin immunofluorescence intensity calculated across CHO cells (dashed lines) or along neurites overlap. Spectrin immunofluorescence intensity relative to nontransfected cells in the same field or to GFP only–transfected cells (n > 30) is significantly higher in NCAM-transfected (C) CHO cells and (D) hippocampal neurons. (E) Levels of spectrin were increased in brain homogenates from wild-type versus NCAM-deficient mice, as assayed by immunoblotting with antibodies against spectrin. Mean values ± SEM from five independent experiments are shown. AU, arbitrary units. *, P < 0.05 (paired t test). Bars: (low power) 20 μm; (high power) 5 μm.
Figure 2.
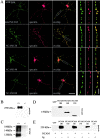
NCAM180, NCAM140, and NCAM120 form complexes with spectrin. (A) Hippocampal neurons from wild-type mice or NCAM−/− mice transfected with NCAM120, NCAM140, or NCAM180 were incubated with NCAM antibodies to induce surface clustering. Note the overlap of NCAM clusters with spectrin. Bars: (low power) 30 μm; (high power) 5 μm. (B) Brain homogenate from wild-type (+/+) or NCAM-deficient (−/−) mice immunoprecipitated with NCAM antibodies and assayed for spectrin by immunoblotting. (C) Brain homogenate from wild-type or NCAM−/− mice immunoprecipitated with spectrin antibodies and assayed for NCAM by immunoblotting. (D) Lysates of CHO cells transfected with GFP, NCAM180, NCAM140, or NCAM120 and assayed for NCAM by immunoblotting. (E) Lysates of transfected CHO cells immunoprecipitated with NCAM antibodies (NCAM) or rabbit IgG (Ig) and assayed for spectrin by immunoblotting. Note the coprecipitation of spectrin with each of the NCAM isoforms.
Figure 3.
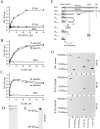
The intracellular (IC) domains of NCAM180 and NCAM140 interact with the NH2-terminal region of spectrin. (A–C) Increasing concentrations of the IC domain of NCAM180 or NCAM140 were bound to plastic and assayed by ELISA for their ability to bind heterodimeric human erythrocyte spectrin (αIβI) or isolated αI and βI spectrin subunits. Binding to BSA served as a control. Mean values ± SEM from six independent experiments are shown. Note that heterodimeric spectrin bound well to IC180, whereas the isolated subunits bound to both IC180 and IC140. (D) Coomassie blue staining of the purified αIβI dimers and the isolated αI and βI spectrin subunits. (E) Isolated brain membranes assayed for βI spectrin by immunoblotting after PAGE under nondenaturing conditions. Note the low molecular weight band representing βI spectrin monomers. (F) Schematic alignment of βI spectrin fragments. For comparison, the βIΣ1 and βIΣ2 isoforms of βI spectrin are shown. These differ by alternative mRNA splicing at their COOH terminus, which contains the pleckstrin homology (PH) domain. ABD, actin-binding domain; MAD1, membrane association domain 1; ANK, ankyrin-binding domain. (G) Lysates of CHO cells transfected with NCAM180 or NCAM140 together with FLAG-labeled spectrin fragments were immunoblotted with FLAG antibodies to confirm approximately equal expression of each of the constructs (top). Lysates from NCAM180 (middle)– and NCAM140 (bottom)–transfected cells were immunoprecipitated (IP) with NCAM antibodies and immunoblotted with FLAG antibodies to detect which of the spectrin constructs would precipitate. Note that only βIN-5 and βI2–3 are present in the NCAM precipitates.
Figure 4.
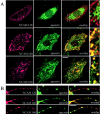
NCAM120 interacts with spectrin through rafts. Double immunostaining of (A) CHO cells and (B) hippocampal neurons from NCAM−/− mice transfected with NCAM120, NCAM140, or NCAM180 with antibodies against NCAM and spectrin after treatment with MCD. Note that MCD disrupts the association of spectrin with NCAM120, but not with NCAM140 or NCAM180. Bars: (low power) 10 μm; (high power) 5 μm. (C) Immunoblots of lysates from transfected CHO cells reveal comparable NCAM isoform expression. GFP-transfected cells do not express NCAM. (D) Lysates from NCAM-transfected CHO cells were precipitated with NCAM antibodies (NCAM) or rabbit IgG (Ig) and assayed for spectrin by immunoblotting. Note that MCD treatment prevents the precipitation of spectrin with NCAM120, but not with NCAM140 or NCAM180.
Figure 4.
NCAM120 interacts with spectrin through rafts. Double immunostaining of (A) CHO cells and (B) hippocampal neurons from NCAM−/− mice transfected with NCAM120, NCAM140, or NCAM180 with antibodies against NCAM and spectrin after treatment with MCD. Note that MCD disrupts the association of spectrin with NCAM120, but not with NCAM140 or NCAM180. Bars: (low power) 10 μm; (high power) 5 μm. (C) Immunoblots of lysates from transfected CHO cells reveal comparable NCAM isoform expression. GFP-transfected cells do not express NCAM. (D) Lysates from NCAM-transfected CHO cells were precipitated with NCAM antibodies (NCAM) or rabbit IgG (Ig) and assayed for spectrin by immunoblotting. Note that MCD treatment prevents the precipitation of spectrin with NCAM120, but not with NCAM140 or NCAM180.
Figure 4.
NCAM120 interacts with spectrin through rafts. Double immunostaining of (A) CHO cells and (B) hippocampal neurons from NCAM−/− mice transfected with NCAM120, NCAM140, or NCAM180 with antibodies against NCAM and spectrin after treatment with MCD. Note that MCD disrupts the association of spectrin with NCAM120, but not with NCAM140 or NCAM180. Bars: (low power) 10 μm; (high power) 5 μm. (C) Immunoblots of lysates from transfected CHO cells reveal comparable NCAM isoform expression. GFP-transfected cells do not express NCAM. (D) Lysates from NCAM-transfected CHO cells were precipitated with NCAM antibodies (NCAM) or rabbit IgG (Ig) and assayed for spectrin by immunoblotting. Note that MCD treatment prevents the precipitation of spectrin with NCAM120, but not with NCAM140 or NCAM180.
Figure 5.
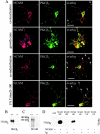
Complexes containing NCAM120 and spectrin occur predominantly within rafts, whereas complexes with NCAM140 or NCAM180 and spectrin are present both within and outside of rafts. (A) Double immunostaining for NCAM and spectrin in CHO cells transfected with NCAM120, NCAM140, or NCAM180 and extracted with 1% Triton X-100. Note the coincidence of spectrin with detergent-insoluble NCAM clusters. Bars: (low power) 20 μm; (high power) 5 μm. (B) Triple labeling for NCAM, spectrin, and GM1 in neurons from NCAM−/− mice transfected with NCAM120, NCAM140, or NCAM180 and extracted with 1% Triton X-100. Note that complexes of NCAM120 and spectrin overlap with ganglioside GM1, whereas NCAM140 and NCAM180 clusters overlap with spectrin clusters in both GM1-positive and GM1-free areas. Bar, 10 μm. (C–E) NCAM-positive clusters in transfected neurons were outlined, and mean fluorescence intensities of NCAM (C), GM1 (D), and spectrin (E) were measured within the outlines. NCAM-negative GM1 clusters from NCAM140- and NCAM180-transfected neurons were taken as a reference value (C–E, 4). Note that all NCAM isoforms are associated with GM1-positive areas (D, compare 4 with 3, showing that NCAM120 localizes predominantly to GM1-positive rafts; also compare 4 with 1 and 2, showing that some NCAM180 and NCAM140 localizes to GM1-positive rafts). Spectrin is enriched in NCAM clusters (E). AU, arbitrary units. Mean values ± SEM are shown (n > 100). *, P < 0.05 (paired t test).
Figure 6.
NCAM and PKCβ2 are colocalized in detergent-insoluble clusters in neurons. (A) Hippocampal neurons stained with antibodies to NCAM and PKCβ2. Clusters of NCAM colocalize with accumulations of PKCβ2 along neurites and in growth cones (colocalization, arrows). In growth cones, NCAM clusters overlap with PKCβ2 accumulations (growth cone). Clustering of NCAM by antibodies induce coredistribution of PKCβ2 with NCAM clusters (coredistribution, arrows). In neurons extracted with detergent (1% Triton X-100), clusters of NCAM overlap with detergent-insoluble PKCβ2 (arrows). Bars: (rows 1, 3, and 4) 20 μm; (row 2) 10 μm. (B) Brain homogenates from wild-type (+/+) or NCAM-deficient (−/−) mice were immunoprecipitated with NCAM antibodies and assayed for PKCβ2 by immunoblotting. (C) Brain homogenate from wild-type (+/+) or NCAM-deficient (−/−) mice immunoprecipitated with PKCβ2 antibodies and assayed for NCAM by immunoblotting with NCAM antibodies. (D) Lysates of CHO cells transfected with NCAM180, NCAM140, or NCAM120 were immunoprecipitated with NCAM antibodies (NCAM) or rabbit IgG (Ig) and probed for PKCβ2 by immunoblotting. Note that PKCβ2 is precipitated with NCAM180 and NCAM140, but not with NCAM120.
Figure 7.
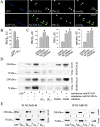
NCAM activation promotes formation of NCAM–spectrin–PKCβ2 complexes and enhances their association with lipid rafts. (A) Triple labeling for NCAM, GM1, and spectrin or PKCβ2 in neurons from wild-type mice extracted with 1% Triton X-100. Note the increase in intensity of spectrin, PKCβ2, and GM1 labeling (arrows) in NCAM clusters after preincubation of cells with NCAM antibodies (30 min) when compared with nonstimulated (control) cells. Images were taken with the same settings. Bar, 10 μm. (B) NCAM clusters were outlined, and mean fluorescence intensities of GM1 and spectrin or PKCβ2 were measured within the outlines for control cells and neurons stimulated with NCAM-Fc or NCAM antibodies. GM1, spectrin, and PKCβ2 are enriched in NCAM clusters after NCAM activation both with NCAM-Fc and NCAM antibodies. Mean values ± SEM are shown (n > 50 neurites). *, P < 0.05 (paired t test). (C and D) Immunoblot of NCAM in four raft fractions isolated by equilibrium sucrose gradient centrifugation from total brain (C) or growth cones (D). Mean values of PKC enzyme activity ± SEM are shown for each fraction derived from six independent experiments. PKC activity correlates with the presence of NCAM140 and NCAM180.
Figure 7.
NCAM activation promotes formation of NCAM–spectrin–PKCβ2 complexes and enhances their association with lipid rafts. (A) Triple labeling for NCAM, GM1, and spectrin or PKCβ2 in neurons from wild-type mice extracted with 1% Triton X-100. Note the increase in intensity of spectrin, PKCβ2, and GM1 labeling (arrows) in NCAM clusters after preincubation of cells with NCAM antibodies (30 min) when compared with nonstimulated (control) cells. Images were taken with the same settings. Bar, 10 μm. (B) NCAM clusters were outlined, and mean fluorescence intensities of GM1 and spectrin or PKCβ2 were measured within the outlines for control cells and neurons stimulated with NCAM-Fc or NCAM antibodies. GM1, spectrin, and PKCβ2 are enriched in NCAM clusters after NCAM activation both with NCAM-Fc and NCAM antibodies. Mean values ± SEM are shown (n > 50 neurites). *, P < 0.05 (paired t test). (C and D) Immunoblot of NCAM in four raft fractions isolated by equilibrium sucrose gradient centrifugation from total brain (C) or growth cones (D). Mean values of PKC enzyme activity ± SEM are shown for each fraction derived from six independent experiments. PKC activity correlates with the presence of NCAM140 and NCAM180.
Figure 7.
NCAM activation promotes formation of NCAM–spectrin–PKCβ2 complexes and enhances their association with lipid rafts. (A) Triple labeling for NCAM, GM1, and spectrin or PKCβ2 in neurons from wild-type mice extracted with 1% Triton X-100. Note the increase in intensity of spectrin, PKCβ2, and GM1 labeling (arrows) in NCAM clusters after preincubation of cells with NCAM antibodies (30 min) when compared with nonstimulated (control) cells. Images were taken with the same settings. Bar, 10 μm. (B) NCAM clusters were outlined, and mean fluorescence intensities of GM1 and spectrin or PKCβ2 were measured within the outlines for control cells and neurons stimulated with NCAM-Fc or NCAM antibodies. GM1, spectrin, and PKCβ2 are enriched in NCAM clusters after NCAM activation both with NCAM-Fc and NCAM antibodies. Mean values ± SEM are shown (n > 50 neurites). *, P < 0.05 (paired t test). (C and D) Immunoblot of NCAM in four raft fractions isolated by equilibrium sucrose gradient centrifugation from total brain (C) or growth cones (D). Mean values of PKC enzyme activity ± SEM are shown for each fraction derived from six independent experiments. PKC activity correlates with the presence of NCAM140 and NCAM180.
Figure 8.
NCAM140 and NCAM180 bind PKCβ2 via spectrin in an FGFR- dependent manner. (A) Wild-type neurons were transfected either with GFP or GFP together with the βI2–3 construct. NCAM was clustered by application of NCAM antibodies to live cells. Note the reduced redistribution of PKCβ2 to NCAM clusters in neurons cotransfected with βI2–3. Bar, 10 μm. NCAM clusters were outlined, and the level of PKCβ2 immunoreactivity was measured within the outlines (B). Mean values ± SEM are shown (n > 20 neurites). (C) Hippocampal neurons were incubated with NCAM antibodies or NCAM antibodies plus the FGFR inhibitor (PD173074, 50 nM), extracted with 1% Triton X-100, and triple labeled for NCAM, GM1, PKCβ2, or spectrin. NCAM clusters were outlined, and the mean fluorescence intensities of GM1 and PKCβ2 or spectrin were measured within the outlines for control cells and neurons stimulated with NCAM antibodies with or without the inhibitor. The FGFR inhibitor blocked redistribution of PKCβ2 to NCAM clusters but not recruitment of spectrin to NCAM and redistribution of the NCAM–spectrin complex to lipid rafts. Mean values ± SEM are shown (n > 50 neurites). *, P < 0.05 (paired t test). (D) CHO cells transfected with GFP, NCAM140, NCAM180, NCAM140 + βI2–3, or NCAM180 + βI2–3 were incubated with NCAM-Fc or NCAM-Fc plus the FGFR inhibitor. Lysates were immunoprecipitated with NCAM antibodies and probed for spectrin and PKCβ2 by immunoblotting. Fragment βI2–3 interferes with the precipitation of spectrin and PKCβ2, whereas the FGFR inhibitor blocks only precipitation of PKCβ2. Note the increased amount of spectrin and PKCβ2 precipitating with NCAM after NCAM activation. (E) Lysates of CHO cells cotransfected with NCAM140 or NCAM180 with GFP, βIN-5, βIN,3–5, βI2–3, or βIN-2 were immunoprecipitated with NCAM antibodies and probed for spectrin and PKCβ2 by immunoblotting. Note that only fragments βIN-5 and βI2–3 interfere with the precipitation of spectrin and PKCβ2.
Figure 9.
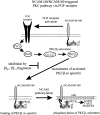
Proposed model of NCAM–spectrin–PKC interactions in the NCAM-triggered PKC pathway. See text in Discussion.
Similar articles
- RPTPalpha is essential for NCAM-mediated p59fyn activation and neurite elongation.
Bodrikov V, Leshchyns'ka I, Sytnyk V, Overvoorde J, den Hertog J, Schachner M. Bodrikov V, et al. J Cell Biol. 2005 Jan 3;168(1):127-39. doi: 10.1083/jcb.200405073. Epub 2004 Dec 28. J Cell Biol. 2005. PMID: 15623578 Free PMC article. - The neural cell adhesion molecule regulates cell-surface delivery of G-protein-activated inwardly rectifying potassium channels via lipid rafts.
Delling M, Wischmeyer E, Dityatev A, Sytnyk V, Veh RW, Karschin A, Schachner M. Delling M, et al. J Neurosci. 2002 Aug 15;22(16):7154-64. doi: 10.1523/JNEUROSCI.22-16-07154.2002. J Neurosci. 2002. PMID: 12177211 Free PMC article. - Cosignaling of NCAM via lipid rafts and the FGF receptor is required for neuritogenesis.
Niethammer P, Delling M, Sytnyk V, Dityatev A, Fukami K, Schachner M. Niethammer P, et al. J Cell Biol. 2002 Apr 29;157(3):521-32. doi: 10.1083/jcb.200109059. Epub 2002 Apr 29. J Cell Biol. 2002. PMID: 11980923 Free PMC article. - Role of the growth-associated protein GAP-43 in NCAM-mediated neurite outgrowth.
Korshunova I, Mosevitsky M. Korshunova I, et al. Adv Exp Med Biol. 2010;663:169-82. doi: 10.1007/978-1-4419-1170-4_11. Adv Exp Med Biol. 2010. PMID: 20017022 Review. No abstract available. - The neural cell adhesion molecule NCAM and lipid rafts.
Povlsen GK, Ditlevsen DK. Povlsen GK, et al. Adv Exp Med Biol. 2010;663:183-98. doi: 10.1007/978-1-4419-1170-4_12. Adv Exp Med Biol. 2010. PMID: 20017023 Review. No abstract available.
Cited by
- Neural cell adhesion molecule 2 promotes the formation of filopodia and neurite branching by inducing submembrane increases in Ca2+ levels.
Sheng L, Leshchyns'ka I, Sytnyk V. Sheng L, et al. J Neurosci. 2015 Jan 28;35(4):1739-52. doi: 10.1523/JNEUROSCI.1714-14.2015. J Neurosci. 2015. PMID: 25632147 Free PMC article. - Targeted deletion of betaIII spectrin impairs synaptogenesis and generates ataxic and seizure phenotypes.
Stankewich MC, Gwynn B, Ardito T, Ji L, Kim J, Robledo RF, Lux SE, Peters LL, Morrow JS. Stankewich MC, et al. Proc Natl Acad Sci U S A. 2010 Mar 30;107(13):6022-7. doi: 10.1073/pnas.1001522107. Epub 2010 Mar 15. Proc Natl Acad Sci U S A. 2010. PMID: 20231455 Free PMC article. - RPTPalpha is essential for NCAM-mediated p59fyn activation and neurite elongation.
Bodrikov V, Leshchyns'ka I, Sytnyk V, Overvoorde J, den Hertog J, Schachner M. Bodrikov V, et al. J Cell Biol. 2005 Jan 3;168(1):127-39. doi: 10.1083/jcb.200405073. Epub 2004 Dec 28. J Cell Biol. 2005. PMID: 15623578 Free PMC article. - The neural cell adhesion molecule promotes maturation of the presynaptic endocytotic machinery by switching synaptic vesicle recycling from adaptor protein 3 (AP-3)- to AP-2-dependent mechanisms.
Shetty A, Sytnyk V, Leshchyns'ka I, Puchkov D, Haucke V, Schachner M. Shetty A, et al. J Neurosci. 2013 Oct 16;33(42):16828-45. doi: 10.1523/JNEUROSCI.2192-13.2013. J Neurosci. 2013. PMID: 24133283 Free PMC article. - The loss of βΙ spectrin alters synaptic size and composition in the ja/ja mouse.
Stankewich MC, Peters LL, Morrow JS. Stankewich MC, et al. Front Neurosci. 2024 Aug 6;18:1415115. doi: 10.3389/fnins.2024.1415115. eCollection 2024. Front Neurosci. 2024. PMID: 39165342 Free PMC article.
References
- Aarts, L.H., P. Verkade, J.J. van Dalen, A.J. van Rozen, W.H. Gispen, L.H. Schrama, and P. Schotman. 1999. B-50/GAP-43 potentiates cytoskeletal reorganization in raft domains. Mol. Cell. Neurosci. 14:85–97. - PubMed
- Beggs, H.E., S.C. Baragona, J.J. Hemperly, and P.F. Maness. 1997. NCAM140 interacts with the focal adhesion kinase p125(fak) and the SRC-related tyrosine kinase p59(fyn). J. Biol. Chem. 272:8310–8319. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous