Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice - PubMed (original) (raw)
Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice
Takehiro Nitta et al. J Cell Biol. 2003.
Abstract
Tight junctions are well-developed between adjacent endothelial cells of blood vessels in the central nervous system, and play a central role in establishing the blood-brain barrier (BBB). Claudin-5 is a major cell adhesion molecule of tight junctions in brain endothelial cells. To examine its possible involvement in the BBB, claudin-5-deficient mice were generated. In the brains of these mice, the development and morphology of blood vessels were not altered, showing no bleeding or edema. However, tracer experiments and magnetic resonance imaging revealed that in these mice, the BBB against small molecules (<800 D), but not larger molecules, was selectively affected. This unexpected finding (i.e., the size-selective loosening of the BBB) not only provides new insight into the basic molecular physiology of BBB but also opens a new way to deliver potential drugs across the BBB into the central nervous system.
Figures
Figure 1.
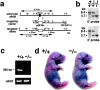
Generation of Cld-5–deficient mice. (a) Restriction maps of the wild-type allele, the targeting vector, and the targeted allele of the mouse Cld-5 gene. Only one exon covers the whole open reading frame of Cld-5. The targeting vector contained the pgk neo cassette in its middle portion to delete the exon in the targeted allele. The positions of the 5′ and 3′ probes for Southern blotting are indicated as bars. E, EcoRI; Sa, SacI; K, KpnI; N, NcoI; and S, Sse8387I. (b) Genotype analyses by Southern blotting of Eco RI-digested genomic DNA from wild-type (+/+), heterozygous (+/−), and homozygous (−/−) mice for the mutant Cld-5 gene allele. Southern blotting with 5′ and 3′ probes yielded an 8.4-kb band from the wild-type allele, and a 4.7- and 3.7-kb band from the targeted allele, respectively. (c) Loss of Cld-5 mRNA in the brain of Cld-5 −/− mice examined by RT-PCR. As a control, the hypoxanthine phosphoribosyl transferase gene was equally amplified in all samples. (d) Newborn Cld-5 +/+ and Cld-5 −/− mice. Homozygous Cld-5–deficient mice were born in the expected Mendelian ratios, and looked normal macroscopically. However, their movements gradually ceased, and they all died within 10 h of birth.
Figure 2.
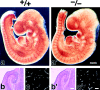
Vasculogenesis and the brain histology of Cld5 −/− mice. (a and a′) Whole-mount immunostaining. Mouse 9.5-d wild-type and Cld5 −/− embryos were labeled with anti–PECAM-1 mAb. The characteristic treelike and ladderlike staining patterns of blood vessels in the head and vertebrate portions were observed both in wild-type and Cld5 −/− embryos. (b, b′, c, and c′) Hematoxylin-eosin–stained paraffin sections (b and b′) and anti–PECAM-1–stained frozen sections (c and c′) of the wild-type and Cld5 −/− brains of 18.5-d embryos. No difference was detected. Bars: (a and a′) 300 μm; (b and b′) 100 μm; (c and c′) 40 μm.
Figure 3.
Ultrastructure of brain blood vessels in Cl d5 −**/**− mice. In the Cld5 −/− brains of 18.5-d embryo, ultrathin-section electron microscopy revealed no overt morphological abnormalities in the blood vessels. TJs were observed between adjacent endothelial cells, and at higher magnification, so-called kissing points of TJs were clearly visualized (arrowheads). Bars: (left) 4 μm; (middle) 0.2 μm; (right) 50 nm.
Figure 4.
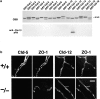
Cld-12 in brain blood vessels. (a) Specificity of newly generated anti–Cld-12 pAb. Immunoblotting of total lysates of Escherichia coli expressing GST fusion proteins with cytoplasmic domains of Cld-1–16 confirmed its specificity. CBB, Coomassie brilliant blue staining; anti–Cld-12 pAb_,_ immunoblotting with anti–Cld-12 pAb. (b) Frozen sections of the wild-type and Cld5 −/− brains of 18.5-d embryo were double stained with anti–ZO-1 mAb and anti–Cld-5 pAb or anti–Cld-12 pAb. Cld-5 was completely undetectable from the Cld5 −/− brain. Cld-12 was concentrated at ZO-1–positive TJs not only in the wild-type but also in the Cld5 −/− brain endothelial cells. These Cld-12 signals were abolished when the anti–Cld-12 pAb was preincubated with the GST-Cld-12 fusion protein. There appeared to be no significant difference in the intensity of Cld-12 signal between the wild-type and Cld5 −/− brain endothelial cells. The concentration of occludin at TJs of endothelial cells was not affected in the Cld5 −/− brain (unpublished data). Bar, 20 μm.
Figure 5.
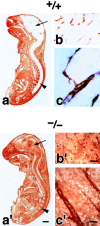
Impairment of the BBB in the Cld5 −**/**− brains of 18.5-d embryos. Primary amine-reactive biotinylation reagent (443 D) was perfused from the heart of resuscitated embryos. After a 5-min incubation, sagittal frozen sections from the whole body (a and a′) or the brain (b, b′, c, and c′) were incubated with HRP-conjugated streptavidin to localize the biotinylation reagent by peroxidase activity. At a low magnification in the wild-type mouse (a), the reagent was completely excluded from the brain (arrow) and the spinal cord (arrowhead); in sharp contrast, in the Cld5 −/− mouse (a′), the CNS showed intense peroxidase activity. At a higher magnification, in the Cld5 −/− (b′ and c′) but not in the wild-type brain (b and c), the reagent infiltrated abundantly into the parenchyma. Bars: (a and a′) 2 mm; (b and b′) 40 μm; (c and c′) 10 μm.
Figure 6.
Time course of the biotinylation reagent leakage from the blood vessels in the Cld5 −**/**− brains. Primary amine-reactive biotinylation reagent (443 D) was perfused from the heart of resuscitated embryos. After a 1, 3, 5, or 10-min incubation, frozen sections from the brain were incubated with rhodamine-conjugated streptavidin to visualize the distribution of the injected biotinylation reagent. Bar, 50 μm.
Figure 7.
Size-selective loosening of the BBB in Cld5 −**/**− mice. (a) Paraffin sections of the brain were de-paraffined and stained with rabbit antialbumin pAb, followed by incubation with HRP-conjugated anti–rabbit IgG pAb. Bound pAb was visualized by peroxidase activity. No leakage of endogenous serum albumin was observed in the Cld5 −/− brain. Bar, 40 μm. (b) Microperoxidase (∼1.9 kD) was injected into the heart of resuscitated embryos, and after a 5-min incubation, the brain was fixed. The localization of microperoxidase was visualized by peroxidase activity in frozen sections. No leakage of microperoxidase was observed in the Cld5 −/− brain. Bar, 40 μm. (c) A mixture of tetramethylrhodamine-conjugated dextran (∼10 kD) and a dye for nuclear staining (Hoechst stain H33258; 562 D) was perfused, and frozen sections were observed under a fluorescence microscope. In the wild-type brain, neither dextran (red) nor Hoechst dye (blue) extravasated, whereas in the Cld5 −/− brain, Hoechst dye, but not dextran, extravasated to stain the nuclei of surrounding neurons/glial cells. Bar, 30 μm.
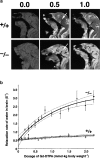
Figure 8.
MRI of 18.5-d embryos. (a) Mid-sagittal T1-weighted images of the wild-type and Cld5 −/− brain after the transcardial injection of Gd-DTPA (0.0, 0.5, and 1.0 mmol/kg body wt). Due to shortening of the T1 relaxation time, the Gd-DTPA–accessible area was represented as a high signal intensity. In the wild-type mice, Gd-DTPA did not enter the extravascular space in the CNS, so the brain parenchyma (top middle, arrow) maintained a constant image intensity even at a high dose of Gd-DTPA. By contrast, in the Cld5 −/− brain, the image intensity in all areas of the CNS (bottom middle, arrow), including the spinal cord, was enhanced by Gd-DTPA, and the degree of enhancement depends on the dosage of injected Gd-DTPA. (b) The relationship between the relaxation rate of water (1/T1) in the brain and the dosage of injected Gd-DTPA. As 1/T1 of the brain, 1/T1 values of the five ROIs (156 × 156 × 750 mm) including the cortex, thalamus, hypothalamus, cerebellum, and pons were averaged. The bold and dotted lines are the results of fitting and 95% confidence limits, respectively. As explained in the text, on the assumption of a simple two-compartment exchange model (Fabry and Eisenstadt, 1978; Schwarzbauer et al., 1997), this relationship allowed us to estimate quantitatively the Gd-DTPA–accessible space per unit volume of the brain.
Comment in
- Holey barrier: claudins and the regulation of brain endothelial permeability.
Matter K, Balda MS. Matter K, et al. J Cell Biol. 2003 May 12;161(3):459-60. doi: 10.1083/jcb.200304039. J Cell Biol. 2003. PMID: 12743096 Free PMC article. Review.
Similar articles
- Holey barrier: claudins and the regulation of brain endothelial permeability.
Matter K, Balda MS. Matter K, et al. J Cell Biol. 2003 May 12;161(3):459-60. doi: 10.1083/jcb.200304039. J Cell Biol. 2003. PMID: 12743096 Free PMC article. Review. - Severe alterations of endothelial and glial cells in the blood-brain barrier of dystrophic mdx mice.
Nico B, Frigeri A, Nicchia GP, Corsi P, Ribatti D, Quondamatteo F, Herken R, Girolamo F, Marzullo A, Svelto M, Roncali L. Nico B, et al. Glia. 2003 May;42(3):235-51. doi: 10.1002/glia.10216. Glia. 2003. PMID: 12673830 - Claudin peptidomimetics modulate tissue barriers for enhanced drug delivery.
Dithmer S, Staat C, Müller C, Ku MC, Pohlmann A, Niendorf T, Gehne N, Fallier-Becker P, Kittel Á, Walter FR, Veszelka S, Deli MA, Blasig R, Haseloff RF, Blasig IE, Winkler L. Dithmer S, et al. Ann N Y Acad Sci. 2017 Jun;1397(1):169-184. doi: 10.1111/nyas.13359. Epub 2017 May 15. Ann N Y Acad Sci. 2017. PMID: 28505395 - Functional effectiveness of the blood-brain barrier to small water-soluble molecules in developing and adult opossum (Monodelphis domestica).
Ek CJ, Dziegielewska KM, Stolp H, Saunders NR. Ek CJ, et al. J Comp Neurol. 2006 May 1;496(1):13-26. doi: 10.1002/cne.20885. J Comp Neurol. 2006. PMID: 16528724 Free PMC article. - Claudins in occluding junctions of humans and flies.
Furuse M, Tsukita S. Furuse M, et al. Trends Cell Biol. 2006 Apr;16(4):181-8. doi: 10.1016/j.tcb.2006.02.006. Epub 2006 Mar 14. Trends Cell Biol. 2006. PMID: 16537104 Review.
Cited by
- GDNF and cAMP significantly enhance in vitro blood-brain barrier integrity in a humanized tricellular transwell model.
Kanjanasirirat P, Saengsawang W, Ketsawatsomkron P, Asavapanumas N, Borwornpinyo S, Soodvilai S, Hongeng S, Charoensutthivarakul S. Kanjanasirirat P, et al. Heliyon. 2024 Oct 12;10(20):e39343. doi: 10.1016/j.heliyon.2024.e39343. eCollection 2024 Oct 30. Heliyon. 2024. PMID: 39492921 Free PMC article. - Aging disrupts blood-brain and blood-spinal cord barrier homeostasis, but does not increase paracellular permeability.
Cummins MJ, Cresswell ET, Bevege RJ, Smith DW. Cummins MJ, et al. Geroscience. 2024 Oct 30. doi: 10.1007/s11357-024-01404-9. Online ahead of print. Geroscience. 2024. PMID: 39476323 - Neurons enhance blood-brain barrier function via upregulating claudin-5 and VE-cadherin expression due to glial cell line-derived neurotrophic factor secretion.
Yang L, Lin Z, Mu R, Wu W, Zhi H, Liu X, Yang H, Liu L. Yang L, et al. Elife. 2024 Oct 30;13:RP96161. doi: 10.7554/eLife.96161. Elife. 2024. PMID: 39475379 Free PMC article. - Endothelial Myosin IIA Is Required for the Maintenance of Blood-Brain Barrier Integrity.
Deng Y, Qiao Z, Zhou C, Pei Y, Xu H, Kang X, Luo J. Deng Y, et al. Cells. 2024 Oct 1;13(19):1635. doi: 10.3390/cells13191635. Cells. 2024. PMID: 39404399 Free PMC article. - Astrocytic DLL4-NOTCH1 signaling pathway promotes neuroinflammation via the IL-6-STAT3 axis.
Mora P, Laisné M, Bourguignon C, Rouault P, Jaspard-Vinassa B, Maître M, Gadeau AP, Renault MA, Horng S, Couffinhal T, Chapouly C. Mora P, et al. J Neuroinflammation. 2024 Oct 10;21(1):258. doi: 10.1186/s12974-024-03246-w. J Neuroinflammation. 2024. PMID: 39390606 Free PMC article.
References
- Abbott, N.J., D.C. Chugani, G. Zaharchuk, and B.R. Rose. 1999. Delivery of imaging agents into brain. Adv. Drug Deliv. Rev. 37:253–277. - PubMed
- Anderson, J.M., and C.M. van Itallie. 1995. Tight junctions and the molecular basis for regulation of paracellular permeability. Am. J. Physiol. 269:G467–G475. - PubMed
- Balda, M.S., and K. Matter. 1998. Tight junctions. J. Cell Sci. 111:541–547. - PubMed
- Balda, M.S., J.A. Whitney, C. Flores, S. González, M. Cereijido, and K. Matter. 1996. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical–basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J. Cell Biol. 134:1031–1049. - PMC - PubMed
- Caravan, P., J.J. Ilison, T.J. McMurry, and R.B. Lauffer. 1999. Gadolinium(III) chelates as MRI contrast agents: structure, dynamics, and applications. Chem. Rev. 99:2293–2352. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous