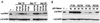Absence of p53 complements defects in Abelson murine leukemia virus signaling - PubMed (original) (raw)
Absence of p53 complements defects in Abelson murine leukemia virus signaling
Indira Unnikrishnan et al. J Virol. 2003 Jun.
Abstract
The v-Abl protein encoded by Abelson murine leukemia virus (Ab-MLV) induces transformation of pre-B cells via a two-stage process. An initial proliferative phase during which cells with limited tumorigenic potential expand is followed by a crisis period marked by high levels of apoptosis and erratic growth. Transformants that survive this phase emerge as fully malignant cells and usually contain mutations that disable the p53 tumor suppressor pathway. Consistent with the importance of p53 in this process, pre-B cells from p53 null animals bypass crisis. Thus, the transformation process reflects a balance between signals from the v-Abl protein that drive transformation and those coming from the cellular response to inappropriate growth. One prediction of this hypothesis is that Ab-MLV mutants that are compromised in their ability to transform cells may be less equipped to overcome the effects of p53. To test this idea, we examined the ability of the P120/R273K mutant to transform pre-B cells from wild-type, p53 null, and Ink4a/Arf null mice. The SH2 domain of the v-Abl protein encoded by this mutant contains a substitution that affects the phosphotyrosine-binding pocket, and this mutant is compromised in its ability to transform NIH 3T3 and pre-B cells, especially at 39.5 degrees C. Our data reveal that loss of p53 or Ink4a/Arf locus products complements the transforming defect of the P120/R273K mutant, but it does not completely restore wild-type function. These results indicate that one important transforming function of v-Abl proteins is overcoming the effects of a functional p53 pathway.
Figures
FIG. 1.
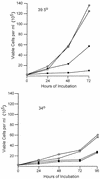
The absence of p53 does not complement the growth defect of transformants expressing P120/R273K. Transformants generated from p53 null mice at 34 and 39.5°C with P120 and P120/R273K were plated at 2.5 × 105 cells per ml in duplicate, and growth and viability were monitored by counting trypan blue-stained samples at regular intervals. Cells expressing P120/R273K are represented by the circles, and cells expressing P120 are represented by the squares. Each line represents an independent cell line. The experiment shown is representative of at least two independent experiments with these cells and of experiments performed with four additional primary transformants derived using either P120 or P120/R273K. Cell numbers in duplicate cultures varied by <10%.
FIG. 2.
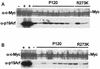
Myc and p19Arf expression is similar in primary transformants expressing P120 and P120/R273K. Primary transformants from wild-type bone marrow expressing P120 or P120/R273K were plated in liquid medium and lysed at the stage of primary transformation immediately after explant from agar (A) or 1 day later (B). The lysates were analyzed by using Western blotting and anti-p19Arf and anti-Myc antibodies. Each lane represents an independent sample. Controls included fully established Ab-MLV-transformed cell lines that express abundant p19Arf (+) and a fully transformed Ab-MLV cell line derived from an Ink4a/Arf null mouse (−) (18).
FIG. 3.
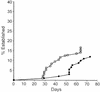
P120/R273K primary transformants require longer to establish. Primary transformants were prepared by infecting bone marrow from wild-type mice with either P120 or P120/R273K at 37°C, and the cells were removed from agar and plated in liquid medium (day 0). Growth and viability were monitored, and the cultures were considered established when the cells divided predictably and displayed low levels of apoptosis (12, 18, 27). Each point represents a single culture. The squares represent P120-infected cells; the circles represent P120/R273K-infected cells.
FIG. 4.
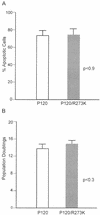
Differences in growth rate, but not apoptosis, influence crisis duration. (A) The average number of apoptotic cells in primary transformants was monitored by flow cytometry. The data shown represent analyses of 10 independent P120-infected primary transformants and 7 independent P120/R273K transformants. Similar results were obtained with samples analyzed at two other time points during crisis. (B) The average number of population doublings required for cells to transit crisis was calculated. The calculations shown represent analysis of growth information for all of the primary transformants illustrated in Fig. 2. The error bars indicate standard errors of the means.
FIG. 5.
Patterns of p53 mutation and p19Arf expression are similar during crisis and in fully established P120- and P120/R273K-derived transformants. (A) Cells undergoing crisis were lysed and analyzed by Western blotting using anti-p19Arf and anti-β-actin antibodies. The days indicate the time after explant from agar; each lane represents an independent sample. (B) Fully transformed cell lines were analyzed by using Western blotting and anti-p19Arf and anti-β-actin antibodies. The samples shown are representative of the cell lines described in Table 4. M, mutant p53; W, wild-type p53. In both panels, controls included a fully established Ab-MLV-transformed cell line that expresses mutant p53 and abundant p19Arf (+) and a fully transformed Ab-MLV cell line derived from an Ink4a/Arf null mouse (−).
Similar articles
- Active Akt and functional p53 modulate apoptosis in Abelson virus-transformed pre-B cells.
Gong L, Unnikrishnan I, Raghavan A, Parmar K, Rosenberg N. Gong L, et al. J Virol. 2004 Feb;78(4):1636-44. doi: 10.1128/jvi.78.4.1636-1644.2004. J Virol. 2004. PMID: 14747529 Free PMC article. - Temperature-sensitive transformation by an Abelson virus mutant encoding an altered SH2 domain.
Mainville CA, Parmar K, Unnikrishnan I, Gong L, Raffel GD, Rosenberg N. Mainville CA, et al. J Virol. 2001 Feb;75(4):1816-23. doi: 10.1128/JVI.75.4.1816-1823.2001. J Virol. 2001. PMID: 11160680 Free PMC article. - The carboxyl terminus of v-Abl protein can augment SH2 domain function.
Warren D, Heilpern AJ, Berg K, Rosenberg N. Warren D, et al. J Virol. 2000 May;74(10):4495-504. doi: 10.1128/jvi.74.10.4495-4504.2000. J Virol. 2000. PMID: 10775585 Free PMC article. - Transforming pathways activated by the v-Abl tyrosine kinase.
Shore SK, Tantravahi RV, Reddy EP. Shore SK, et al. Oncogene. 2002 Dec 9;21(56):8568-76. doi: 10.1038/sj.onc.1206084. Oncogene. 2002. PMID: 12476303 Review. - Inhibition of Abelson oncogene function by erbstatin analogues.
Kawada M, Tawara J, Tsuji T, Honma Y, Hozumi M, Wang JY, Umezawa K. Kawada M, et al. Drugs Exp Clin Res. 1993;19(6):235-41. Drugs Exp Clin Res. 1993. PMID: 8013266 Review.
Cited by
- Active Akt and functional p53 modulate apoptosis in Abelson virus-transformed pre-B cells.
Gong L, Unnikrishnan I, Raghavan A, Parmar K, Rosenberg N. Gong L, et al. J Virol. 2004 Feb;78(4):1636-44. doi: 10.1128/jvi.78.4.1636-1644.2004. J Virol. 2004. PMID: 14747529 Free PMC article. - Rapid, stabilizing palindrome rearrangements in somatic cells by the center-break mechanism.
Cunningham LA, Coté AG, Cam-Ozdemir C, Lewis SM. Cunningham LA, et al. Mol Cell Biol. 2003 Dec;23(23):8740-50. doi: 10.1128/MCB.23.23.8740-8750.2003. Mol Cell Biol. 2003. PMID: 14612414 Free PMC article. - SH2-containing inositol 5'-phosphatase inhibits transformation of Abelson murine leukemia virus.
Fessler SP, Rosenberg N, Baughn LB. Fessler SP, et al. J Virol. 2011 Sep;85(17):9239-42. doi: 10.1128/JVI.05115-11. Epub 2011 Jun 22. J Virol. 2011. PMID: 21697469 Free PMC article.
References
- Bradshaw, J. M., V. Mitaxov, and G. Waksman. 1999. Investigation of phosphotyrosine recognition by the SH2 domain of the Src kinase. J. Mol. Biol. 293:971-985. - PubMed
- Bradshaw, J. M., V. Mitaxov, and G. Waksman. 2000. Mutational investigation of the specificity determining region of the Src SH2 domain. J. Mol. Biol. 299:521-535. - PubMed
- Chen, Y.-Y., L. C. Wang, M. S. Huang, and N. Rosenberg. 1994. An active v-abl protein tyrosine kinase blocks immunoglobulin light-chain gene rearrangement. Genes Dev. 8:688-697. - PubMed
- Danial, N. N., and P. Rothman. 2000. JAK-STAT signaling activated by Abl oncogenes. Oncogene 19:2523-2531. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous