Cellular gene expression survey of vaccinia virus infection of human HeLa cells - PubMed (original) (raw)
Cellular gene expression survey of vaccinia virus infection of human HeLa cells
Susana Guerra et al. J Virol. 2003 Jun.
Abstract
Vaccinia virus (VV) is a cytocidal virus that causes major changes in host cell machinery shortly after infecting cells. To define the consequences of virus infection on host gene expression, we used microarrays of approximately 15,000 human cDNAs to examine expression levels of mRNAs isolated at 2, 6, and 16 h postinfection from cultures of infected HeLa cells. The majority of profiling changes during VV infection corresponded to downregulation of genes at 16 h postinfection. Differentially expressed genes were clustered into seven groups to identify common regulatory pathways, with most of them (90%) belonging to clusters 6 and 7, which represent genes whose expression was repressed after infection. Cluster 1, however, contained 37 transcripts (2.81%) showing a robust pattern of induction that was maintained during the course of infection. Genes in cluster 1 included those for Wiskott-Aldrich syndrome protein (WASP) family member WASF1, thymosine, adenosine A2a receptor, glutamate decarboxylase 2, CD-80 antigen, KIAA0888 protein, selenophosphate synthetase, pericentrin, and attractin as well as several expressed sequence tags. We analyzed in more detail the fate of WASP protein in VV-infected cells, because a related family member, N-WASP, is involved in viral motility. WASP protein accumulated in the course of infection; its increase required viral DNA replication and de novo protein synthesis, and it localized in cytoplasmic structures distinct from uninfected cells. This study is the first quantitative analysis of host gene expression following VV infection of cultured human cells, demonstrating global changes in the expression profile, and identifies upregulated genes with potential roles in the virus replication cycle.
Figures
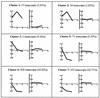
FIG. 1.
Representation of 10 by 5 map obtained by the self-organizing maps algorithm, showing the gene expression clusters for VV-infected HeLa cells. Experimental points on the x axis are indicated as 1 for 2 h, 2 for 6 h, and 3 for 16 h postinfection. The y axis shows normalized expression values. Each cluster depicted was numbered from 1 to 7.
FIG. 2.
Characteristic expression patterns represented in each cluster. Mean values (left) and standard deviations (right) of the expression profiles of genes assigned to each cluster. Experimental points on the x axis are as in Fig. 1. The y axis shows normalized expression values. Cluster 4 was not included because it contains only one transcript (0.08%).
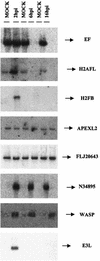
FIG.3.
Validation of microarray data by Northern blot. Total RNA (20 μg) purified from uninfected and VV-infected cells at 2, 6, and 16 h postinfection was hybridized with probes derived from PCR products that were spotted on the microarray. The genes included in the autoradiogram are N34895 (EST), WASP (Wiskott-Aldrich syndrome protein), H2AFL (histone L), H2FB (histone B), EF (elongation factor), APEXL2 (apurinic/apyridiminic endonuclease), FLJ20643 (EST), and E3L (VV protein).
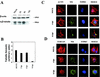
FIG. 4.
Changes in cytoskeletal components after VV infection. (A) Western blot comparison of actin and tubulin protein levels in lysates of mock- and VV-infected cells. Total proteins (100 μg) were separated by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-β-actin and anti-β-tubulin antibodies. (B) Densitometric quantification of tubulin protein. (C) Immunofluorescence analysis of the effect of VV infection on actin fibers in HeLa cells. Mock- and VV-infected cells at 6 and 16 h postinfection (hpi) were labeled with monoclonal antibody C3α14k to detect the A27L viral p14 protein, followed by the appropriate phalloidin-conjugated secondary antibody and ToPro reagent. The samples were analyzed by confocal immunofluorescence microscopy. (D) Immunofluorescence analysis of the effect of VV infection on tubulin.
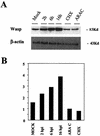
FIG. 5.
Upregulation of WASP protein after VV infection. (A) Comparison of WASP protein levels by Western blot from lysates of mock- and VV-infected cells at 2, 6, and 16 h postinfection (hpi) and from cells treated at infection with cycloheximide (CHX; 100 μg/ml) and adenosine arabinoside (ARAC; 50 μg/ml) for 16 h. Total proteins (100 μg) were separated by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-WASP and anti-β-actin antibodies. (B) Densitometric quantification of WASP protein.
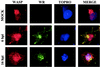
FIG. 6.
Immunofluorescence analysis showing WASP redistribution in VV-infected HeLa cells. Mock- and VV-infected cells at 6 and 16 h postinfection (hpi) were double-labeled with monoclonal antibody C3α14k to detect the A27L viral p14 protein and anti-WASP antibody, followed by the appropriate fluorescent secondary antibody and ToPro reagent. Cells were visualized by confocal immunofluorescence microscopy.
FIG. 7.
VV infection changes the subcellular localization of WASP from the Golgi complex. HeLa cells cultured on coverslips were infected with VV for 6 and 16 h. Cells were treated with anti-WASP antibody, followed by an appropriate fluorescent secondary antibody, ToPro, and fluorescent anti-wheat germ antigen (WGA).
Similar articles
- Wiskott-Aldrich syndrome protein is needed for vaccinia virus pathogenesis.
Guerra S, Aracil M, Conde R, Bernad A, Esteban M. Guerra S, et al. J Virol. 2005 Feb;79(4):2133-40. doi: 10.1128/JVI.79.4.2133-2140.2005. J Virol. 2005. PMID: 15681416 Free PMC article. - Microarray analysis reveals characteristic changes of host cell gene expression in response to attenuated modified vaccinia virus Ankara infection of human HeLa cells.
Guerra S, López-Fernández LA, Conde R, Pascual-Montano A, Harshman K, Esteban M. Guerra S, et al. J Virol. 2004 Jun;78(11):5820-34. doi: 10.1128/JVI.78.11.5820-5834.2004. J Virol. 2004. PMID: 15140980 Free PMC article. - Increased ATP generation in the host cell is required for efficient vaccinia virus production.
Chang CW, Li HC, Hsu CF, Chang CY, Lo SY. Chang CW, et al. J Biomed Sci. 2009 Sep 2;16(1):80. doi: 10.1186/1423-0127-16-80. J Biomed Sci. 2009. PMID: 19725950 Free PMC article. - A role for the small GTPase Rac1 in vaccinia actin-based motility.
Alvarez DE, Agaisse H. Alvarez DE, et al. Small GTPases. 2015;6(2):119-22. doi: 10.1080/21541248.2015.1055182. Small GTPases. 2015. PMID: 26147090 Free PMC article. Review. - Analysis of host responses to microbial infection using gene expression profiling.
Kagnoff MF, Eckmann L. Kagnoff MF, et al. Curr Opin Microbiol. 2001 Jun;4(3):246-50. doi: 10.1016/s1369-5274(00)00198-3. Curr Opin Microbiol. 2001. PMID: 11378474 Review.
Cited by
- A Combined Transcriptomic and Proteomic Analysis of Monkeypox Virus A23 Protein on HEK293T Cells.
Wang Y, Li Y, Li M, Wang K, Xiong J, Wang T, Wang Y, Guo Y, Kong L, Li M. Wang Y, et al. Int J Mol Sci. 2024 Aug 8;25(16):8678. doi: 10.3390/ijms25168678. Int J Mol Sci. 2024. PMID: 39201364 Free PMC article. - CK2 phosphorylation of CMTR1 promotes RNA cap formation and influenza virus infection.
Lukoszek R, Inesta-Vaquera F, Brett NJM, Liang S, Hepburn LA, Hughes DJ, Pirillo C, Roberts EW, Cowling VH. Lukoszek R, et al. Cell Rep. 2024 Jul 23;43(7):114405. doi: 10.1016/j.celrep.2024.114405. Epub 2024 Jun 25. Cell Rep. 2024. PMID: 38923463 Free PMC article. - ISG15 Is Required for the Dissemination of Vaccinia Virus Extracellular Virions.
Bécares M, Albert M, Tárrega C, Coloma R, Falqui M, Luhmann EK, Radoshevich L, Guerra S. Bécares M, et al. Microbiol Spectr. 2023 Jun 15;11(3):e0450822. doi: 10.1128/spectrum.04508-22. Epub 2023 Apr 10. Microbiol Spectr. 2023. PMID: 37036376 Free PMC article. - Vaccinia Virus Arrests and Shifts the Cell Cycle.
Martin CK, Samolej J, Olson AT, Bertoli C, Wiebe MS, de Bruin RAM, Mercer J. Martin CK, et al. Viruses. 2022 Feb 19;14(2):431. doi: 10.3390/v14020431. Viruses. 2022. PMID: 35216024 Free PMC article. - CMTR1 is recruited to transcription start sites and promotes ribosomal protein and histone gene expression in embryonic stem cells.
Liang S, Silva JC, Suska O, Lukoszek R, Almohammed R, Cowling VH. Liang S, et al. Nucleic Acids Res. 2022 Mar 21;50(5):2905-2922. doi: 10.1093/nar/gkac122. Nucleic Acids Res. 2022. PMID: 35212377 Free PMC article.
References
- Adams, C., P. Diadori, L. Schoenroth, and M. Fritzler. 2000. Autoantibodies in childhood post-varicella acute cerebellar ataxia. Can. J. Neurol. Sci. 4:316-320. - PubMed
- Bablanian, R., B. Baxt, J. A. Sonnabend, and M. Esteban. 1978. Studies on the mechanism of vaccinia virus cytopathic effects. II. Early cell rounding is associated with virus polypeptide synthesis. J. Gen. Virol. 39:403-413. - PubMed
- Bablanian, R., G. Coppola, S. Scribani, and M. Esteban. 1981. Inhibition of protein synthesis by vaccinia virus. IV. The role of low-molecular-weight viral RNA in the inhibition of protein synthesis. Virology 112:13-24. - PubMed
- Bablanian, R., M. Esteban, B. Bax, and J. A. Sonnabend. 1978. Studies on the mechanisms of vaccinia virus cytopathic effects. I. Inhibition of protein synthesis in infected cells is associated with virus-induced RNA synthesis. J. Gen. Virol. 39:391-402. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases