The addicted human brain: insights from imaging studies - PubMed (original) (raw)
Review
The addicted human brain: insights from imaging studies
Nora D Volkow et al. J Clin Invest. 2003 May.
No abstract available
Figures
Figure 1
Drugs of abuse have effects at multiple biological and environmental levels. The environmental level is identified as “social,” since this is the most relevant of the environmental factors that influence drug abuse in humans. Imaging techniques allow one to assess the effects of drugs of abuse at the protein and the brain circuit levels and to assess how these effects relate to behavior. Imaging also offers a way to start to assess the impact of environmental factors on these biological levels, as well as the impact of gene polymorphisms on protein expression and brain function.
Figure 2
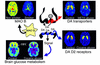
Images obtained with PET (axial sections) that show the effects of chronic drug exposure on various proteins involved in dopamine (DA) neurotransmission and on brain function (as assessed by brain glucose metabolism). While some effects are common to many drugs of abuse, such as decreases in DA D2 receptors in striatal neurons and decreased metabolic activity in the orbitofrontal cortex (OFC), others are more specific. These include the decrease in DA transporters in striatum in methamphetamine (METH) abusers (possibly the result of neurotoxicity to DA terminals) and the decrease in brain monoamine oxidase B (MAO B; the enzyme involved in DA metabolism) in cigarette smokers. The rainbow scale was used to code the PET images; radiotracer concentration is displayed from higher to lower as red > yellow > green > blue. Images from methamphetamine use are adapted from ref. . Images from smokers are adapted with permission from ref. .
Figure 3
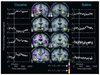
Images of coronal sections obtained with fMRI, showing areas of brain activation and deactivation during cocaine intoxication compared with those after saline administration. During intoxication there is a complex pattern of activation and/or deactivation that includes the ventral tegmental area (VTA) and the substantia nigra (SN), where DA cells are located, as well as regions involved with reward (nucleus accumbens, NAc; basal forebrain, BF; globus pallidus, GP), with memory (amygdala), and with motivation (subcallosal cortex, SCC). The color scale indicates the level of significance (P value) of the change in activation of the bold signal. Reproduced with permission from Neuron (9).
Figure 4
Images of axial sections obtained with PET, showing DA D2 receptors in nonhuman primates that were initially tested while housed in separate cages and then retested after being housed in a group. Animals that became dominant when placed in a group (a) showed increased numbers of DA D2 receptors in striatum, whereas subordinate animals (b) did not. (c) The levels of cocaine administration in the subordinate and the dominant animals. Note the much lower intake of cocaine by dominant animals which possessed higher numbers of DA D2 receptors. The temperature scale was used to code the PET images; radiotracer concentration is displayed from higher to lower as yellow > red. Asterisks indicate significant differences in drug intake between groups. Adapted with permission from ref. .
Figure 5
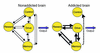
Model proposing a network of four circuits involved with addiction: reward, motivation/drive, memory, and control. These circuits work together and change with experience. Each is linked to an important concept: saliency (reward), internal state (motivation/drive), learned associations (memory), and conflict resolution (control). During addiction, the enhanced value of the drug in the reward, motivation, and memory circuits overcomes the inhibitory control exerted by the prefrontal cortex, thereby favoring a positive-feedback loop initiated by the consumption of the drug and perpetuated by the enhanced activation of the motivation/drive and memory circuits.
Figure 6
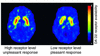
Images of axial sections obtained with PET to measure the numbers of DA D2 receptors in subjects who reported the effects of the stimulant drug methylphenidate as pleasant versus those that reported its effects as unpleasant. Subjects with high numbers of DA D2 receptors tended to report the effects of methylphenidate as unpleasant, whereas subjects with low numbers of DA D2 receptors tended to report it as pleasant. The rainbow scale was used to code the PET images; radiotracer concentration is displayed from higher to lower as red > yellow > green > blue. Adapted with permission from ref. .
Similar articles
- Addiction and imaging of the living human brain.
Gatley SJ, Volkow ND. Gatley SJ, et al. Drug Alcohol Depend. 1998 Jun-Jul;51(1-2):97-108. doi: 10.1016/s0376-8716(98)00069-6. Drug Alcohol Depend. 1998. PMID: 9716933 Review. No abstract available. - Reward mechanisms in the brain and their role in dependence: evidence from neurophysiological and neuroimaging studies.
Martin-Soelch C, Leenders KL, Chevalley AF, Missimer J, Künig G, Magyar S, Mino A, Schultz W. Martin-Soelch C, et al. Brain Res Brain Res Rev. 2001 Oct;36(2-3):139-49. doi: 10.1016/s0165-0173(01)00089-3. Brain Res Brain Res Rev. 2001. PMID: 11690610 Review. - Behavioral perspectives on the neuroscience of drug addiction.
Winger G, Woods JH, Galuska CM, Wade-Galuska T. Winger G, et al. J Exp Anal Behav. 2005 Nov;84(3):667-81. doi: 10.1901/jeab.2005.101-04. J Exp Anal Behav. 2005. PMID: 16596985 Free PMC article. Review. - Positron emission tomography and single-photon emission computed tomography in substance abuse research.
Volkow ND, Fowler JS, Wang GJ. Volkow ND, et al. Semin Nucl Med. 2003 Apr;33(2):114-28. doi: 10.1053/snuc.2003.127300. Semin Nucl Med. 2003. PMID: 12756644 Review. - Human brain imaging and substance abuse.
Lingford-Hughes A. Lingford-Hughes A. Curr Opin Pharmacol. 2005 Feb;5(1):42-6. doi: 10.1016/j.coph.2004.10.002. Curr Opin Pharmacol. 2005. PMID: 15661624 Review.
Cited by
- Neural cue-reactivity in pathological gambling as evidence for behavioral addiction: a systematic review.
García-Castro J, Cancela A, Cárdaba MAM. García-Castro J, et al. Curr Psychol. 2022 Nov 4:1-12. doi: 10.1007/s12144-022-03915-0. Online ahead of print. Curr Psychol. 2022. PMID: 36373116 Free PMC article. - Emerging roles of actin cytoskeleton regulating enzymes in drug addiction: actin or reactin'?
Rothenfluh A, Cowan CW. Rothenfluh A, et al. Curr Opin Neurobiol. 2013 Aug;23(4):507-12. doi: 10.1016/j.conb.2013.01.027. Epub 2013 Feb 18. Curr Opin Neurobiol. 2013. PMID: 23428655 Free PMC article. Review. - Up-regulation of CB1 cannabinoid receptors located at glutamatergic terminals in the medial prefrontal cortex of the obese Zucker rat.
Echeazarra L, Barrondo S, García Del Caño G, Bonilla-Del Río I, Egaña-Huguet J, Puente N, Aretxabala X, Montaña M, López de Jesús M, González-Burguera I, Saumell-Esnaola M, Goicolea MA, Grandes P, Sallés J. Echeazarra L, et al. Front Neuroanat. 2022 Oct 18;16:1004702. doi: 10.3389/fnana.2022.1004702. eCollection 2022. Front Neuroanat. 2022. PMID: 36329829 Free PMC article. - Perceived social support in patients with chronic pain with and without opioid use disorder and role of medication for opioid use disorder.
Benville JR, Compton P, Giordano NA, Cheatle MD. Benville JR, et al. Drug Alcohol Depend. 2021 Apr 1;221:108619. doi: 10.1016/j.drugalcdep.2021.108619. Epub 2021 Feb 15. Drug Alcohol Depend. 2021. PMID: 33667781 Free PMC article. - Work engagement, impulsivity and, self-efficacy among Polish workers. Moderating role of impulsivity.
Rożnowski B, Wontorczyk A. Rożnowski B, et al. PLoS One. 2024 Oct 10;19(10):e0311856. doi: 10.1371/journal.pone.0311856. eCollection 2024. PLoS One. 2024. PMID: 39388452 Free PMC article.
References
- Leshner AI. Addiction is a brain disease, and it matters. Science. 1997;278:45–47. - PubMed
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat. Rev. Neurosci. 2001;2:119–128. - PubMed
- Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat. Rev. Neurosci. 2001;2:695–703. - PubMed
- Koob GF, Bloom FE. Cellular and molecular mechanism of drug dependence. Science. 1988;242:715–723. - PubMed