Intrinsic light responses of retinal ganglion cells projecting to the circadian system - PubMed (original) (raw)
Intrinsic light responses of retinal ganglion cells projecting to the circadian system
Erin J Warren et al. Eur J Neurosci. 2003 May.
Abstract
In mammals, light entrainment of the circadian clock, located in the suprachiasmatic nuclei (SCN), requires retinal input. Traditional rod and cone photoreceptors, however, are not required. Instead, the SCN-projecting retinal ganglion cells (RGCs) function as autonomous photoreceptors and exhibit light responses independent of rod- and cone-driven input. Using whole-cell patch-clamp recording techniques, we have investigated the morphological and electrophysiological properties of this unique class of RGCs. Although SCN-projecting RGCs resemble Type III cells in form, they display strikingly different physiological properties from these neurons. First, in response to the injection of a sustained depolarizing current, SCN-projecting cells fired in a transient fashion, in contrast to most RGCs which fired robust trains of action potentials. Second, in response to light, SCN-projecting RGCs exhibited an intensity-dependent transient depolarization in the absence of rod and cone input. This depolarization reached a peak within 5 s and generated increased spiking activity before decaying to a plateau. Voltage-clamp recordings were used to characterize the light-activated conductance which generated this depolarization. In response to varying light intensities, SCN-projecting RGCs exhibited a graded transient inward current which peaked within 5 s and decayed to a plateau. The voltage dependence of the light-activated current was obtained by subtracting currents elicited by a voltage ramp before and during illumination. The light-activated current displayed both inward and outward rectification and was largely unaffected by substitution of extracellular Na+ with choline. In both respects, the intrinsic light-activated current observed in SCN-projecting RGCs resembles currents carried by ion channels of the transient receptor potential (trp) family, which are known to mediate the light response of invertebrate photoreceptors.
Figures
FIG. 1
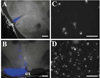
Retrograde labelling of RGCs following stereotaxic injection of the SCN. (A) 200-μm brain slice containing the central SCN was imaged using DIC optics and overlaid with a fluorescence image (blue) showing the location of the fluorescent beads; ox, optic chiasm. (B) Similar overlay showing an injection into the optic nerve. (C and D) Photomicrographs of retinal wholemounts showing the distribution of labelled neurons resulting from injection of (C) the SCN and (D) the optic nerve, respectively. Scale bars, 500 μm (A and B), 25 μm (C and D).
FIG. 2
Morphology of SCN-projecting neurons. (A) Photomicrograph showing the typical morphology of SCN-projecting RGCs. Filled cell was visualized with a DAB reaction and photographed. (B) A 2-D representation of the same neuron using the Neurolucida software. (C) Cross-sectional representations of three Neurolucida reconstructed neurons to show the variety of stratification observed in SCN-projecting RGCs. (D) A polar plot of the neuron from A and B, used to determine dendritic polarity index and dendritic distribution index. Scale bars, 100 μm (A and B), 12.5, 20 and 25 μm (C, from top).
FIG. 3
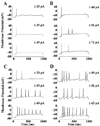
Spiking patterns of SCN-projecting RGCs. (A and B) Current-clamp recordings from two SCN-projecting RGCs. The response of each cell to increasing depolarization is shown from bottom to top. The magnitude of the depolarization is shown on the right. (C and D) For comparison, the spiking patterns elicited from a similar stimulus protocol are shown for (C) a Type I and (D) a Type II neuron.
FIG. 4
Intrinsic light responses of SCN-projecting RGCs. During recordings, cells were exposed to a 20-s step of white light from a tungsten-halogen lamp (bar). (A) In current-clamp, the cells responded to light (5.8 × 10−5W/cm2) with a slow depolarization which generated increased spiking activity. (B) In contrast the resting membrane activity of unlabelled ganglion cells, chosen at random, did not show any response to the same light stimulus (top two traces). No light responses were observed from RGCs labelled by an optic nerve hit (lower trace). (C) The responses of an SCN-projecting RGC to increasingly brighter illumination. The numbers below each trace represent the light intensity of the stimulus in W/cm2. (D) The relationship between stimulus intensity and spike frequency was approximately linear over the range of light intensities used.
FIG. 5
Current–voltage relationship of the light-activated current. (A) The lower panel shows the light response (black bar; irradiance 5.8×10−5W/cm2) of an SCN-projecting RGC recorded in voltage-clamp mode. To illustrate the similar time courses, a light response recorded in current-clamp mode is shown in the upper panel. (B)To determine the voltage dependence of the light-activated current, voltage ramps (−100 to +100 mVover 2 s) were applied before and during illumination. (C) The light-activated current (red) was determined by subtracting currents elicited by the voltage ramp before (black) and during (blue) illumination.
FIG. 6
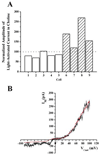
The light-activated current was insensitive to replacement of extracellular Na+. (A) The bars represent the amplitude of the light-activated current recorded in an external solution containing 20mM (grey) or 0mM (hatched) Na+. The amplitudes in low Na+ were normalized to the amplitude of the current recorded in 120mM Na+ extracellular solution. The permutation test for paired differences indicates that the amplitude of the light sensitive current in low Na+ was not significantly different (P = 0.125) from that measured in normal Na+ extracellular solution. (B) I–V characteristics of the light-activated current in 120mM Na+ (black) and 0 Na+ (red). The I–V characteristic in 0 Na+ was obtained first and, to permit comparison, has been scaled to match the amplitude of the current obtained at +100mV in 120mM Na+.
Similar articles
- The light-activated signaling pathway in SCN-projecting rat retinal ganglion cells.
Warren EJ, Allen CN, Brown RL, Robinson DW. Warren EJ, et al. Eur J Neurosci. 2006 May;23(9):2477-87. doi: 10.1111/j.1460-9568.2006.04777.x. Eur J Neurosci. 2006. PMID: 16706854 Free PMC article. - Synaptic inputs to retinal ganglion cells that set the circadian clock.
Perez-Leon JA, Warren EJ, Allen CN, Robinson DW, Brown RL. Perez-Leon JA, et al. Eur J Neurosci. 2006 Aug;24(4):1117-23. doi: 10.1111/j.1460-9568.2006.04999.x. Eur J Neurosci. 2006. PMID: 16930437 Free PMC article. - A broad role for melanopsin in nonvisual photoreception.
Gooley JJ, Lu J, Fischer D, Saper CB. Gooley JJ, et al. J Neurosci. 2003 Aug 6;23(18):7093-106. doi: 10.1523/JNEUROSCI.23-18-07093.2003. J Neurosci. 2003. PMID: 12904470 Free PMC article. - Intrinsically photosensitive retinal ganglion cells.
Pickard GE, Sollars PJ. Pickard GE, et al. Sci China Life Sci. 2010 Jan;53(1):58-67. doi: 10.1007/s11427-010-0024-5. Epub 2010 Feb 12. Sci China Life Sci. 2010. PMID: 20596956 Review. - The Neurobiology of Circadian Rhythms.
Sollars PJ, Pickard GE. Sollars PJ, et al. Psychiatr Clin North Am. 2015 Dec;38(4):645-65. doi: 10.1016/j.psc.2015.07.003. Epub 2015 Sep 5. Psychiatr Clin North Am. 2015. PMID: 26600101 Free PMC article. Review.
Cited by
- Melanopsin and inner retinal photoreception.
Bailes HJ, Lucas RJ. Bailes HJ, et al. Cell Mol Life Sci. 2010 Jan;67(1):99-111. doi: 10.1007/s00018-009-0155-7. Epub 2009 Oct 29. Cell Mol Life Sci. 2010. PMID: 19865798 Free PMC article. Review. - Light-evoked calcium responses of isolated melanopsin-expressing retinal ganglion cells.
Hartwick AT, Bramley JR, Yu J, Stevens KT, Allen CN, Baldridge WH, Sollars PJ, Pickard GE. Hartwick AT, et al. J Neurosci. 2007 Dec 5;27(49):13468-80. doi: 10.1523/JNEUROSCI.3626-07.2007. J Neurosci. 2007. PMID: 18057205 Free PMC article. - Linking neural activity and molecular oscillations in the SCN.
Colwell CS. Colwell CS. Nat Rev Neurosci. 2011 Sep 2;12(10):553-69. doi: 10.1038/nrn3086. Nat Rev Neurosci. 2011. PMID: 21886186 Free PMC article. Review. - Photochemistry of retinal chromophore in mouse melanopsin.
Walker MT, Brown RL, Cronin TW, Robinson PR. Walker MT, et al. Proc Natl Acad Sci U S A. 2008 Jul 1;105(26):8861-5. doi: 10.1073/pnas.0711397105. Epub 2008 Jun 25. Proc Natl Acad Sci U S A. 2008. PMID: 18579788 Free PMC article. - Role of vasoactive intestinal peptide in the light input to the circadian system.
Vosko A, van Diepen HC, Kuljis D, Chiu AM, Heyer D, Terra H, Carpenter E, Michel S, Meijer JH, Colwell CS. Vosko A, et al. Eur J Neurosci. 2015 Jul;42(2):1839-48. doi: 10.1111/ejn.12919. Epub 2015 May 25. Eur J Neurosci. 2015. PMID: 25885685 Free PMC article.
References
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. - PubMed
- Clapham DE, Runnels LW, Struübing C. The TRP ion channel family. Nature Rev. Neurosci. 2001;2:387–396. - PubMed
- Dunn FA, Berson DM. 2002 Annual Meeting. Fort Lauderdale FL: Association for Research in Vision and Ophthamology; 2002. Are intrinisically photosensitive retinal ganglion cells influenced by rods and cones? Abstract and Program Planner Accessed at <Www.Arvo.Org> Abstract number 2982.
Publication types
MeSH terms
Grants and funding
- R21 MH063364-01/MH/NIMH NIH HHS/United States
- MH 63364/MH/NIMH NIH HHS/United States
- R01 MH067094-01A1/MH/NIMH NIH HHS/United States
- R21 MH063364-02/MH/NIMH NIH HHS/United States
- NS07466/NS/NINDS NIH HHS/United States
- R01 MH067094/MH/NIMH NIH HHS/United States
- T32 NS007466/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources