How clonal is Staphylococcus aureus? - PubMed (original) (raw)
. 2003 Jun;185(11):3307-16.
doi: 10.1128/JB.185.11.3307-3316.2003.
Jessica E Cooper, Hajo Grundmann, D Ashley Robinson, Mark C Enright, Tony Berendt, Sharon J Peacock, John Maynard Smith, Michael Murphy, Brian G Spratt, Catrin E Moore, Nicholas P J Day
Affiliations
- PMID: 12754228
- PMCID: PMC155367
- DOI: 10.1128/JB.185.11.3307-3316.2003
How clonal is Staphylococcus aureus?
Edward J Feil et al. J Bacteriol. 2003 Jun.
Abstract
Staphylococcus aureus is an important human pathogen and represents a growing public health burden owing to the emergence and spread of antibiotic-resistant clones, particularly within the hospital environment. Despite this, basic questions about the evolution and population biology of the species, particularly with regard to the extent and impact of homologous recombination, remain unanswered. We address these issues through an analysis of sequence data obtained from the characterization by multilocus sequence typing (MLST) of 334 isolates of S. aureus, recovered from a well-defined population, over a limited time span. We find no significant differences in the distribution of multilocus genotypes between strains isolated from carriers and those from patients with invasive disease; there is, therefore, no evidence from MLST data, which index variation within the stable "core" genome, for the existence of hypervirulent clones of this pathogen. Examination of the sequence changes at MLST loci during clonal diversification shows that point mutations give rise to new alleles at least 15-fold more frequently than does recombination. This contrasts with the naturally transformable species Neisseria meningitidis and Streptococcus pneumoniae, in which alleles change between 5- and 10-fold more frequently by recombination than by mutation. However, phylogenetic analysis suggests that homologous recombination does contribute toward the evolution of this species over the long term. Finally, we note a striking excess of nonsynonymous substitutions in comparisons between isolates belonging to the same clonal complex compared to isolates belonging to different clonal complexes, suggesting that the removal of deleterious mutations by purifying selection may be relatively slow.
Figures
FIG. 1.
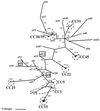
Identification of clonal complexes. BURST analysis was used to assign clonal complexes within the Oxford isolate collection. Splits graphs are also shown for each major clonal complex, generated by Splitstree version 3.1 (no splits graphs are shown for the minor groups). For the major clonal complexes, the predicted clonal ancestors are shown in the central ring, SLVs are shown in the middle (solid) ring, and double-locus variants are shown in the outer (dashed) ring. Individual relationships between some STs are shown as a solid line (single-locus difference) or a dashed line (double-locus difference). The values in parentheses are the numbers of isolates of a given ST when more than one example of an ST was observed.
FIG. 2.
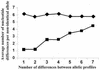
Sequence diversity versus allelic diversity. The average number of nucleotide differences per locus (excluding those loci that do not differ) was calculated (y axis) for all pairwise comparisons of the 75 S. aureus STs (squares) and for the 575 S. pneumoniae STs (diamonds). These averages were computed separately for all pairwise comparisons differing at one, two, three, four, five, six, or all seven loci (x axis). There is a clear positive trend between the number of nucleotide differences in nonidentical alleles and the number of allelic differences (i.e., time since divergence) within the S. aureus data, suggesting that recombination has not been sufficiently frequent to overwhelm the stepwise accumulation of diversification through de novo point mutation. However, this trend is not noted within the data from S. pneumoniae.
FIG. 3.
Unrooted Bayesian tree of the concatenated sequences of the 75 different STs of S. aureus. The tree was constructed by using MrBayes version 2.01 (14); six different substitution rates were estimated with site-specific rate variation. Four Markov chains were run for 1,000,000 generations, and the tree was saved (with branch lengths) every 100 generations, resulting in 10,000 trees. These trees were imported into PAUP* 4.0b10 (33), and the first 2,000 trees were discarded as burn-in. A 50% majority rule consensus tree was computed from these trees, and the posterior probability given on each branch is a percentage of these trees supporting each node. All the major clonal complexes are enclosed within dashed rings; singletons or minor groups represented by more than five strains are boxed. With the exception of ST188 the individual STs within each major clonal complex are not labeled.
FIG. 4.
ML trees. Trees are shown for each MLST gene (a) and for the concatenated sequences of all seven genes (b), with a sample of 25 diverse STs. Each tree was computed by using the HKY85 model of nucleotide substitution, with the α parameter (describing the shape of the gamma distribution of rate variation with eight discrete categories) and the Ti/Tv ratio optimized during tree construction. Despite the presence of multiple inconsistencies between these trees, there is evidence for a conserved division, which is marked with an arrow in each tree. STs belonging to one side of this division (group 1) are given in white on a black background. There are some exceptions to this division, particularly at the arcC locus (see text). The branching order of the concatenated tree (b) is very similar to Fig. 3, which was computed by a Bayesian approach, the main difference being the positions of ST17 and ST59.
Similar articles
- A link between virulence and ecological abundance in natural populations of Staphylococcus aureus.
Day NP, Moore CE, Enright MC, Berendt AR, Smith JM, Murphy MF, Peacock SJ, Spratt BG, Feil EJ. Day NP, et al. Science. 2001 Apr 6;292(5514):114-6. doi: 10.1126/science.1056495. Science. 2001. PMID: 11292876 Retracted. - Inferring a population structure for Staphylococcus epidermidis from multilocus sequence typing data.
Miragaia M, Thomas JC, Couto I, Enright MC, de Lencastre H. Miragaia M, et al. J Bacteriol. 2007 Mar;189(6):2540-52. doi: 10.1128/JB.01484-06. Epub 2007 Jan 12. J Bacteriol. 2007. PMID: 17220222 Free PMC article. - Molecular genetic typing reveals further insights into the diversity of animal-associated Staphylococcus aureus.
Smyth DS, Feil EJ, Meaney WJ, Hartigan PJ, Tollersrud T, Fitzgerald JR, Enright MC, Smyth CJ. Smyth DS, et al. J Med Microbiol. 2009 Oct;58(Pt 10):1343-1353. doi: 10.1099/jmm.0.009837-0. Epub 2009 Jun 15. J Med Microbiol. 2009. PMID: 19528163 - Analyses of clonality and the evolution of bacterial pathogens.
Feil EJ, Enright MC. Feil EJ, et al. Curr Opin Microbiol. 2004 Jun;7(3):308-13. doi: 10.1016/j.mib.2004.04.002. Curr Opin Microbiol. 2004. PMID: 15196500 Review. - Estimating the relative contributions of mutation and recombination to clonal diversification: a comparison between Neisseria meningitidis and Streptococcus pneumoniae.
Feil EJ, Enright MC, Spratt BG. Feil EJ, et al. Res Microbiol. 2000 Jul-Aug;151(6):465-9. doi: 10.1016/s0923-2508(00)00168-6. Res Microbiol. 2000. PMID: 10961460 Review.
Cited by
- Clonally related methicillin-resistant Staphylococcus aureus isolated from short-finned pilot whales (Globicephala macrorhynchus), human volunteers, and a bayfront cetacean rehabilitation facility.
Hower S, Phillips MC, Brodsky M, Dameron A, Tamargo MA, Salazar NC, Jackson CR, Barrett JB, Davidson M, Davis J, Mukherjee S, Ewing RY, Gidley ML, Sinigalliano CD, Johns L, Johnson FE 3rd, Adebanjo O, Plano LR. Hower S, et al. Microb Ecol. 2013 May;65(4):1024-38. doi: 10.1007/s00248-013-0178-3. Epub 2013 Mar 19. Microb Ecol. 2013. PMID: 23508733 - Diversification and Distribution of Ruminant Chlamydia abortus Clones Assessed by MLST and MLVA.
Siarkou VI, Vorimore F, Vicari N, Magnino S, Rodolakis A, Pannekoek Y, Sachse K, Longbottom D, Laroucau K. Siarkou VI, et al. PLoS One. 2015 May 22;10(5):e0126433. doi: 10.1371/journal.pone.0126433. eCollection 2015. PLoS One. 2015. PMID: 26001070 Free PMC article. - Reversions mask the contribution of adaptive evolution in microbiomes.
Torrillo PA, Lieberman TD. Torrillo PA, et al. Elife. 2024 Sep 6;13:e93146. doi: 10.7554/eLife.93146. Elife. 2024. PMID: 39240756 Free PMC article. - No change in the distribution of types and antibiotic resistance in clinical Staphylococcus aureus isolates from orthopaedic patients during a period of 12 years.
Aamot HV, Stavem K, Skråmm I. Aamot HV, et al. Eur J Clin Microbiol Infect Dis. 2015 Sep;34(9):1833-7. doi: 10.1007/s10096-015-2420-z. Epub 2015 Jun 16. Eur J Clin Microbiol Infect Dis. 2015. PMID: 26076750 - Comparative host specificity of human- and pig- associated Staphylococcus aureus clonal lineages.
Moodley A, Espinosa-Gongora C, Nielsen SS, McCarthy AJ, Lindsay JA, Guardabassi L. Moodley A, et al. PLoS One. 2012;7(11):e49344. doi: 10.1371/journal.pone.0049344. Epub 2012 Nov 14. PLoS One. 2012. PMID: 23166643 Free PMC article.
References
- Brueggemann, A. B., D. T. Griffiths, E. Meats, T. Peto, D. W. Crook, and B. G. Spratt. Clonal relationships between invasive and carriage Streptococcus pneumoniae, and serotype- and clone-specific differences in invasive disease potential. J. Infect. Dis., in press. - PubMed
- Day, N. P., C. E. Moore, M. C. Enright, A. R. Berendt, J. M. Smith, M. F. Murphy, S. J. Peacock, B. G. Spratt, and E. J. Feil. 2001. A link between virulence and ecological abundance in natural populations of Staphylococcus aureus. Science 292:114-116. (Retraction, 295:971, 2002.) - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical