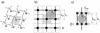Tertiary structure of bacterial murein: the scaffold model - PubMed (original) (raw)
Tertiary structure of bacterial murein: the scaffold model
Boris A Dmitriev et al. J Bacteriol. 2003 Jun.
Abstract
Although the chemical structure and physical properties of peptidoglycan have been elucidated for some time, the precise three-dimensional organization of murein has remained elusive. Earlier published computer simulations of the bacterial murein architecture modeled peptidoglycan strands in either a regular (D. Pink, J. Moeller, B. Quinn, M. Jericho, and T. Beveridge, J. Bacteriol. 182: 5925-5930, 2000) or an irregular (A. Koch, J. Theor. Biol. 204: 533-541, 2000) parallel orientation with respect to the plasma membrane. However, after integrating published experimental data on glycan chain length distribution and the degree of peptide side chain cross-linking into this computer simulation, we now report that the proposed planar network of murein appears largely dysfunctional. In contrast, a scaffold model of murein architecture, which assumes that glycan strands extend perpendicularly to the plasma membrane, was found to accommodate published experimental evidence and yield a viable stress-bearing matrix. Moreover, this model is in accordance with the well-established principle of murein assembly in vivo, i.e., sequential attachment of strands to the preexisting structure. For the first time, the phenomenon of division plane alternation in dividing bacteria can be reconciled with a computer model of the molecular architecture of murein.
Figures
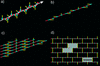
FIG. 1.
Schematic representations of peptidoglycan strand conformation and of a planar murein network. (a) The chain of MurNAc (blue items) and GlcNAc (red items) forms an extended helix. Peptide side chains (yellow bars) project from the helix in a clockwise manner and are perpendicular to each other. (b) Further simplification: the glycan chain is represented as a straight line. (c) Fragment of a murein network composed of long peptidoglycan chains cross-linked to each other via peptide bridges (yellow bars) in a horizontal orientation, whereas vertical peptides remain free. The ratio of bridged to free peptides is 1:1; therefore, the maximal degree of cross-linking is 50%. (d) The most simplified representation of the murein network: the top view includes solely glycan chains and peptide bridges. One tessera and the sample aggregate of two tesserae are shown in gray. The cleavage of long chains (i.e., the existence of short chains) and peptide bridges (i.e., a low degree of cross-linking) will result in the formation of a loose network. Therefore, both the chain length distribution and the degree of cross-linking are critical parameters for the planar murein network.
FIG. 2.
The principle of murein fabric organization according to the scaffold model. The vertical helices are octasaccharide fragments of glycan chains composed of _N_-acetylglucosamine (red items) and muramic acid (blue items) residues. Each strand is oriented along the z axis and is able to cross-link with four others to produce a porous matrix. Yellow sticks represent peptide bridges.
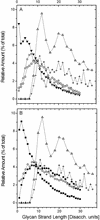
FIG. 3.
Relative amount of glycan strands of length L plotted against glycan strand length L. Amounts indicated by Δ and + in panels A and B were obtained in this study by computer simulation for strands with an even number of disaccharides (Disacch.) or odd and even numbers of disaccharides, respectively, on the basis of data derived from reference . Experimental data obtained from E. coli in panel A: ⋄, strain _fts_Z84 grown at 42°C (31); ○, strain EH3247 (27); ▾, strain MC4100 (45). Experimental data obtained from E. coli strain P678-54 (45) in panel B: •, cylindrical murein; ○, spherical murein; ▪, whole-cell murein.
FIG. 4.
Fragment of the murein matrix simulated in accordance with the scaffold model. (a) Panoramic view of the simulated murein matrix composed of 25 × 25 = 625 positions. The whole matrix contained 625 strands in the functional zone (average length, 11.6 disaccharides) and 470 strands in the degradation zone (average length, 2.9 disaccharides). A narrow and highly cross-linked stress-bearing zone is apparent in the lower part of the matrix. (b) Side view of a fragment of the matrix. The functional zone (zone 1) is composed of peptidoglycan strands comprising 4 to 30 disaccharide-peptide units; anhydromuramic acid residues are directed to the inner membrane. The degradation zone (zone 2) contains recently degraded strands in proximity to the outer membrane. The functional zone, which comprises both assembled and mature murein, includes a compact and highly cross-linked narrow internal zone (zone 3) with a height of 4 disaccharides; this internal zone functions as a stress-bearing structure of the murein fabric. To ensure a clear distinction between different structural units, the color coding of the fragments is the same as in Fig. 1 and 2. Note that peptide bridges are shown while free peptide side chains are omitted.
FIG. 5.
Geometric parameters of the structural units and pores in the scaffold and planar murein models. (a) Schematic representation of the spatial murein matrix (fragment composed of nine strands) and its projection onto the membrane plane. The gray circles stand for projections of the glycan chains, and the gray square area illustrates a surface portion occupied by one chain. It is important to note that each strand occupies the same surface area as one disaccharide unit does. (b) Projection of a scaffold fragment on the membrane plane. The black circles stand for projections of glycan chains; the gray square corresponds to the area occupied by one chain. D stands for the glycan chain diameter, approximately 1.1 nm; Lbr is the length of a peptide bridge, approximately 4.1 nm; Lc is the distance between strand axes, approximately 5.2 nm. A big gray circle with a radius (Rs) of about 2.6 nm represents a cross section of the channel pore expanded by the turgor pressure in the stretched sacculus in vivo. (c) Top view of the tessera in a planar model. The light and dark gray boxes stand for disaccharide units, and LDS stands for the length of one disaccharide unit, about 1 nm; the other designations are the same as those described above. A big gray circle with a radius (Rr) of about 2 nm represents the pore formed by the tessera within the relaxed sacculus.
FIG. 6.
Comparison of the planar and scaffold models under a condition of 33% cross-linking (top-view projections). (a) Fragment of the planar murein network made of glycan strands arranged in 25 rows. The large (more than four tesserae) holes are depicted in gray, and the number of connected tesserae is given for each such hole. The chains forming a piece of network that lost its connection to the fabric are shown in gray, in contrast to the black chains that represent the network itself. Note that tesserae are shown as squares, in accordance with Fig. 5c and the dimensions given in the text. (b) The low cross-linked murein matrix simulated in accordance with the scaffold model. Average degrees of cross-linking: ∼33% (overall), ∼94% (within the internal zone), and ∼7% (in the degradation zone). The number of connected channels is given for each enlarged hole.
Similar articles
- Tertiary structure of Staphylococcus aureus cell wall murein.
Dmitriev BA, Toukach FV, Holst O, Rietschel ET, Ehlers S. Dmitriev BA, et al. J Bacteriol. 2004 Nov;186(21):7141-8. doi: 10.1128/JB.186.21.7141-7148.2004. J Bacteriol. 2004. PMID: 15489425 Free PMC article. - On the architecture of the gram-negative bacterial murein sacculus.
Pink D, Moeller J, Quinn B, Jericho M, Beveridge T. Pink D, et al. J Bacteriol. 2000 Oct;182(20):5925-30. doi: 10.1128/JB.182.20.5925-5930.2000. J Bacteriol. 2000. PMID: 11004199 Free PMC article. - Layered murein revisited: a fundamentally new concept of bacterial cell wall structure, biogenesis and function.
Dmitriev BA, Ehlers S, Rietschel ET. Dmitriev BA, et al. Med Microbiol Immunol. 1999 Mar;187(3):173-81. doi: 10.1007/s004300050090. Med Microbiol Immunol. 1999. PMID: 10206149 Review. - Conformational and topological aspects of the three-dimensional architecture of bacterial peptidoglycan.
Labischinski H, Barnickel G, Naumann D, Keller P. Labischinski H, et al. Ann Inst Pasteur Microbiol (1985). 1985 Jan-Feb;136A(1):45-50. doi: 10.1016/s0769-2609(85)80020-x. Ann Inst Pasteur Microbiol (1985). 1985. PMID: 4004147 - Murein (peptidoglycan) structure, architecture and biosynthesis in Escherichia coli.
Vollmer W, Bertsche U. Vollmer W, et al. Biochim Biophys Acta. 2008 Sep;1778(9):1714-34. doi: 10.1016/j.bbamem.2007.06.007. Epub 2007 Jun 16. Biochim Biophys Acta. 2008. PMID: 17658458 Review.
Cited by
- Physics of bacterial morphogenesis.
Sun SX, Jiang H. Sun SX, et al. Microbiol Mol Biol Rev. 2011 Dec;75(4):543-65. doi: 10.1128/MMBR.00006-11. Microbiol Mol Biol Rev. 2011. PMID: 22126993 Free PMC article. Review. - Uniformity of glycyl bridge lengths in the mature cell walls of fem mutants of methicillin-resistant Staphylococcus aureus.
Sharif S, Kim SJ, Labischinski H, Chen J, Schaefer J. Sharif S, et al. J Bacteriol. 2013 Apr;195(7):1421-7. doi: 10.1128/JB.01471-12. Epub 2013 Jan 18. J Bacteriol. 2013. PMID: 23335411 Free PMC article. - Bacterial cell curvature through mechanical control of cell growth.
Cabeen MT, Charbon G, Vollmer W, Born P, Ausmees N, Weibel DB, Jacobs-Wagner C. Cabeen MT, et al. EMBO J. 2009 May 6;28(9):1208-19. doi: 10.1038/emboj.2009.61. Epub 2009 Mar 12. EMBO J. 2009. PMID: 19279668 Free PMC article. - Peptidoglycan transformations during Bacillus subtilis sporulation.
Tocheva EI, López-Garrido J, Hughes HV, Fredlund J, Kuru E, Vannieuwenhze MS, Brun YV, Pogliano K, Jensen GJ. Tocheva EI, et al. Mol Microbiol. 2013 May;88(4):673-86. doi: 10.1111/mmi.12201. Epub 2013 Mar 27. Mol Microbiol. 2013. PMID: 23531131 Free PMC article. - Staphylococcus aureus peptidoglycan tertiary structure from carbon-13 spin diffusion.
Sharif S, Singh M, Kim SJ, Schaefer J. Sharif S, et al. J Am Chem Soc. 2009 May 27;131(20):7023-30. doi: 10.1021/ja808971c. J Am Chem Soc. 2009. PMID: 19419167 Free PMC article.
References
- Baddiley, J. 1968. The Leeuwenhoek lecture, 1967. Teichoic acids and the molecular structure of bacterial walls. Proc. R. Soc. Lond. B Biol. Sci. 170:331-348. - PubMed
- Boneca, I. G., Z. H. Huang, D. A. Gage, and A. Tomasz. 2000. Characterization of Staphylococcus aureus cell wall glycan strands: evidence for a new β-N-acetylglucosaminidase activity. J. Biol. Chem. 275:9910-9918. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources