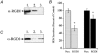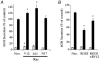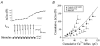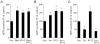Facilitation of Ca(2+)-dependent exocytosis by Rac1-GTPase in bovine chromaffin cells - PubMed (original) (raw)
Facilitation of Ca(2+)-dependent exocytosis by Rac1-GTPase in bovine chromaffin cells
Quanwen Li et al. J Physiol. 2003.
Abstract
Rho family GTPases are primary mediators of cytoskeletal reorganization, although they have also been reported to regulate cell secretion. Yet, the extent to which Rho family GTPases are activated by secretory stimuli in neural and neuroendocrine cells remains unknown. In bovine adrenal chromaffin cells, we found Rac1, but not Cdc42, to be rapidly and selectively activated by secretory stimuli using an assay selective for the activated GTPases. To examine effects of activated Rac1 on secretion, constitutively active mutants of Rac1 (Rac1-V12, Rac1-L61) were transiently expressed in adrenal chromaffin cells. These mutants facilitated secretory responses elicited from populations of intact and digitonin-permeabilized cells as well as from cells under whole cell patch clamp. A dominant negative Rac1 mutant (Rac1-N17) produced no effect on secretion. Expression of RhoGDI, a negative regulator of Rac1, inhibited secretory responses while overexpression of effectors of Rac1, notably, p21-activated kinase (Pak1) and actin depolymerization factor (ADF) promoted evoked secretion. In addition, expression of effector domain mutants of Rac1-V12 that exhibit reduced activation of the cytoskeletal regulators Pak1 and Partner of Rac1 (POR1) resulted in a loss of Rac1-V12-mediated enhancement of evoked secretion. These findings suggest that Rac1, in part, functions to modulate secretion through actions on the cytoskeleton. Consistent with this hypothesis, the actin modifying drugs phalloidin and jasplakinolide enhanced secretion, while latrunculin-A inhibited secretion and eliminated the secretory effects of Rac1-V12. In summary, Rac1 was activated by secretory stimuli and modulated the secretory pathway downstream of Ca2+ influx, partly through regulation of cytoskeletal organization.
Figures
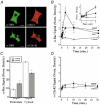
Figure 1. Effect of nicotinic acetylcholine receptor (nAChR) activation and membrane depolarization on the level of GTP-bound Rac1 and Cdc42 in chromaffin cells
A, confocal images showing localization of endogenous α-Rac1 and α-Cdc42 immunoreactivity in chromaffin cells cultured for 3 days. Chromaffin cells were identified by anti-dopamine-β-hydroxylase reactivity as visualized with Alexa488-conjugated secondary antibody. Scale bar = 10 μm. B, time course of Rac1 activation in response to DMPP (20 μM)-mediated nAChR activation (•, n = 5) or elevated K+-induced membrane depolarization (▪, n = 9). Control cells (○, n = 8) from same cell preparations were handled identically, but without stimulation. C, distribution of Rac1 immunoreactivity among cytosol and membrane fractions (particulate) under control conditions and following stimulation with DMPP (20 μM, 2 min, n = 5). D, time course of Cdc42 activation in response to stimulation with DMPP (20 μM; •, n = 7) as compared to control (○, n = 7) cells. Asterisks represent significant differences (P < 0.05) from control.
Figure 2. Transfection and expression of Rac1 mutants in chromaffin cells
A, chromaffin cells were co-transfected with plasmids carrying HA epitope-tagged Rac1 mutants, and a reporter fusion gene (ANP–EmGFP) that is directed to secretory granules of the regulated exocytotic pathway. Co-expression was evaluated by fluorescence detection of the green fluorescent protein and immunofluorescent detection of the HA tag using an α-HA antibody. B, quantification of Rac1 immunofluorescence in non-transfected (control, n = 27) and Rac1-V12 (n = 10)-transfected chromaffin cells. Scale bars = 10 μm.
Figure 3. Effect of co-expression of mutant Rac1 mutants with human growth hormone (hGH) on hGH secretion in permeabilized chromaffin cells
A, time course of hGH secretion from digitonin-permeabilized chromaffin cells transfected with Rac1-V12 (squares) or an empty parent plasmid (Neo., circles). Secretion was elicited by addition of 30 μM free Ca2+ (filled symbols) and compared to secretion in the absence of Ca2+ (open symbols; n = 3). Co-expression of hGH serves as a reporter of the regulated exocytotic pathway of transfected cells. B, effect of expression of Rac1 mutant proteins on Ca2+-evoked hGH secretion at 15 min (n = 9, Rac1-V12 and Rac1-L61; n = 6, Rac1-N17). Data were normalized to control (Neo.) for comparison between different cell preparations. Asterisks represent statistically significant difference (P < 0.05) from control group.
Figure 4. Effect of RhoGDI expression on Ca2+-dependent secretion from digitonin-permeabilized chromaffin cells
A, co-immunoprecipitation of RhoGDI (RGDI) with Rac1 from chromaffin cell lysate. Cell lysates were immunoprecipitated with Rac1 antibody and aliquots of samples subjected to SDS-PAGE and Western blotting. Blots were probed with a RhoGDI. Lane 1, lysate; 2, immunoprecipitate; 3, supernatant of final wash. B, effect of expression of RhoGDI on Ca2+-evoked (30 μM) hGH secretion, measured at 2 min (open bars) and 15 min (filled bars) from permeabilized cells (n = 6). Asterisks represent statistically significant difference (P < 0.05) from control (Neo.). C, dialysis of RhoGDI from permeabilized chromaffin cells. Immunoblot lanes, 1, cell lysate; 2 and 3, aliquot of medium collected from permeabilized chromaffin cells following 2 min and 15 min of Ca2+ stimulation (30 μM), respectively.
Figure 5. Rac1 mutants and RhoGDI alter secretion in intact chromaffin cells
A, effect of constitutively active (V12 and L61) or dominant negative (N17) Rac1 mutants on basal (open bars) and evoked (filled bars) hGH secretion. Control (Neo.) cells were transfected with empty parent plasmid. Secretion was evoked by addition of the nACh receptor agonist DMPP (20 μM, 2 min). B, effect of recombinant RhoGDI expression alone and with Rac1-V12 on basal (open bars) and DMPP-induced (20 μM, 2 min; filled bars) hGH secretion. Asterisks represent statistically significant difference (P < 0.05) from control (Neo.). A, Neo, n = 15; Rac1-V12, n = 15; Rac1-L61, n = 3; Rac1-N17, n = 9; B, Neo, n = 21; RhoGDI, n = 17; RhoGDI+Rac1-V12, n = 18.
Figure 6. Effect of Rac1-V12 and Rho-GDI on the relationship of Ca2+ influx to Δ_C_m
A, whole-cell patch clamp recording from a control ANP–EmGFP transfected cell illustrating evoked _I_Ca and Δ_C_m responses to a train of repetitive step depolarizations (-90 to +10 mV, 50 ms duration). B, comparison of relationships between cumulative Ca2+ influx and cumulative Δ_C_m for control (ANP–EmGFP, filled squares, n = 9), Rac1-V12 (filled circles, n = 9) and recombinant RhoGDI (open circles, n = 4) chromaffin cells. Continuous line represents the standard Ca2+-exocytosis relationship previously described for cultured bovine chromaffin cells (Engisch et al. 1997).
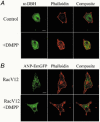
Figure 7. Effect of nACh receptor activation on cortical F-actin in bovine chromaffin cells
A, chromaffin cells were identified by dopamine-β-hydroxylase immunoreactivity and the cortical cytoskeleton visualized by Alexa568-phalloidin staining. A representative example of a control and DMPP-stimulated (20 μM, 2 min) cell is shown. B, visualization of the cortical actin cytoskeleton by Alexa568-phalloidin in Rac1-V12-transfected chromaffin cells under control conditions and following DMPP stimulation (20 μM, 2 min). Chromaffin cells were identified by cotransfection and expression of ANP–EmGFP directed to secretory granules. Scale bar in A and B = 10 μm.
Figure 8. Effects of pharmacological modifiers of F-actin on secretion in transfected chromaffin cells
A, effect of phalloidin on Ca2+-evoked hGH secretion from digitonin-permeabilized chromaffin cells (n = 6). Phalloidin (40 μM) was present during the 2 min permeabilization period and the subsequent 15 min Ca2+ (30 μM) stimulation period. B, representative effect of the membrane-permeant F-actin stabilizing drug jasplakinolide (100 nM) on hGH secretion from intact chromaffin cells stimulated with DMPP (20 μM, 2 min, n = 3). Effect was replicated in 3 separate cell preparations. C, effect of latrunculin-A on Ca2+-evoked hGH secretion from digitonin-permeabilized chromaffin cells (n = 3). Latrunculin-A (10 μM) was present during both the permeabilization period and the 10 min Ca2+ stimulation period. Chromaffin cells were transfected with the constitutively active Rac1 construct (Rac1-V12) or an empty parent plasmid control (Neo.), along with the reporter gene hGH, 3 days prior to initiation of secretion assays.
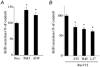
Figure 9. Effects of Rac1 effectors and Rac1-V12 effector domain mutants on secretion in transfected chromaffin cells
A, effect of Pak1 and ADF overexpression on DMPP-induced (20 μM, 2 min) hGH secretion from intact chromaffin cells (n = 6). B, effect of expression of Rac1-V12 and Rac1-V12 effector domain mutants on Ca2+-induced (30 μM; 15 min) hGH secretion from digitonin-permeabilized chromaffin cells (n = 6).
Similar articles
- Involvement of Rho GTPases and their effectors in the secretory process of PC12 cells.
Frantz C, Coppola T, Regazzi R. Frantz C, et al. Exp Cell Res. 2002 Feb 15;273(2):119-26. doi: 10.1006/excr.2001.5432. Exp Cell Res. 2002. PMID: 11822867 - Involvement of Rho and Rac small G proteins and Rho GDI in Ca2+-dependent exocytosis from PC12 cells.
Komuro R, Sasaki T, Takaishi K, Orita S, Takai Y. Komuro R, et al. Genes Cells. 1996 Oct;1(10):943-51. doi: 10.1046/j.1365-2443.1996.760276.x. Genes Cells. 1996. PMID: 9077452 - Vav2 activates Rac1, Cdc42, and RhoA downstream from growth factor receptors but not beta1 integrins.
Liu BP, Burridge K. Liu BP, et al. Mol Cell Biol. 2000 Oct;20(19):7160-9. doi: 10.1128/MCB.20.19.7160-7169.2000. Mol Cell Biol. 2000. PMID: 10982832 Free PMC article. - Emerging Roles of Small GTPases in Islet β-Cell Function.
Veluthakal R, Thurmond DC. Veluthakal R, et al. Cells. 2021 Jun 15;10(6):1503. doi: 10.3390/cells10061503. Cells. 2021. PMID: 34203728 Free PMC article. Review. - Regulation of exocytosis in adrenal chromaffin cells: focus on ARF and Rho GTPases.
Gasman S, Chasserot-Golaz S, Bader MF, Vitale N. Gasman S, et al. Cell Signal. 2003 Oct;15(10):893-9. doi: 10.1016/s0898-6568(03)00052-4. Cell Signal. 2003. PMID: 12873702 Review.
Cited by
- Knee loading reduces MMP13 activity in the mouse cartilage.
Hamamura K, Zhang P, Zhao L, Shim JW, Chen A, Dodge TR, Wan Q, Shih H, Na S, Lin CC, Sun HB, Yokota H. Hamamura K, et al. BMC Musculoskelet Disord. 2013 Nov 1;14:312. doi: 10.1186/1471-2474-14-312. BMC Musculoskelet Disord. 2013. PMID: 24180431 Free PMC article. - Bacterial toxins and the nervous system: neurotoxins and multipotential toxins interacting with neuronal cells.
Popoff MR, Poulain B. Popoff MR, et al. Toxins (Basel). 2010 Apr;2(4):683-737. doi: 10.3390/toxins2040683. Epub 2010 Apr 15. Toxins (Basel). 2010. PMID: 22069606 Free PMC article. Review. - Pleiotropic Roles of Calmodulin in the Regulation of KRas and Rac1 GTPases: Functional Diversity in Health and Disease.
Tebar F, Chavero A, Agell N, Lu A, Rentero C, Enrich C, Grewal T. Tebar F, et al. Int J Mol Sci. 2020 May 23;21(10):3680. doi: 10.3390/ijms21103680. Int J Mol Sci. 2020. PMID: 32456244 Free PMC article. Review. - Cholecystokinin-mediated RhoGDI phosphorylation via PKCα promotes both RhoA and Rac1 signaling.
Sabbatini ME, Williams JA. Sabbatini ME, et al. PLoS One. 2013 Jun 11;8(6):e66029. doi: 10.1371/journal.pone.0066029. Print 2013. PLoS One. 2013. PMID: 23776598 Free PMC article. - Rho GTPases in platelet function.
Aslan JE, McCarty OJ. Aslan JE, et al. J Thromb Haemost. 2013 Jan;11(1):35-46. doi: 10.1111/jth.12051. J Thromb Haemost. 2013. PMID: 23121917 Free PMC article. Review.
References
- Benard V, Bohl BP, Bokoch GM. Characterization of Rac and Cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J Biol Chem. 1999;274:13198–13204. - PubMed
- Boguski MS, McCormick F. Proteins regulating Ras and its relatives. Nature. 1993;366:643–654. - PubMed
- Buchanan FG, Elliot CM, Gibbs M, Exton JH. Translocation of the Rac1 guanine nucleotide exchange factor Tiam1 induced by platelet-derived growth factor and lysophosphatidic acid. J Biol Chem. 2000;275:9742–9748. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM44428/GM/NIGMS NIH HHS/United States
- NS39914/NS/NINDS NIH HHS/United States
- R01 NS039914/NS/NINDS NIH HHS/United States
- R01 GM044428/GM/NIGMS NIH HHS/United States
- R01 DK050127/DK/NIDDK NIH HHS/United States
- DK50127/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous