RNA polymerase mutations that impair conversion to a termination-resistant complex by Q antiterminator proteins - PubMed (original) (raw)
RNA polymerase mutations that impair conversion to a termination-resistant complex by Q antiterminator proteins
Thomas J Santangelo et al. Genes Dev. 2003.
Abstract
Bacteriophage lambda Q-protein stably binds and modifies RNA polymerase (RNAP) to a termination-resistant form. We describe amino acid substitutions in RNAP that disrupt Q-mediated antitermination in vivo and in vitro. The positions of these substitutions in the modeled RNAP/DNA/RNA ternary elongation complex, and their biochemical properties, suggest that they do not define a binding site for Q in RNAP, but instead act by impairing interactions among core RNAP subunits and nucleic acids that are essential for Q modification. A specific conjecture is that Q modification stabilizes interactions of RNAP with the DNA/RNA hybrid and optimizes alignment of the nucleic acids in the catalytic site. Such changes would inhibit the activity of the RNA hairpin of an intrinsic terminator to disrupt the 5'-terminal bases of the hybrid and remove the RNA 3' terminus from the active site.
Figures
Figure 1
Mutant phenotypes. (A) Compilation of RNAP mutants affecting Q activity. Substitutions shown in pairs only diminish Q activity as pairs, and are shown twice to position each bold substitution in the correct spatial group. Mutant classes as discussed in the text are grouped and indicated by background color. β-Gal activity is the average of percent wild-type (% WT) activity at each arabinose concentration. Phage spotting efficiency is relative to the same strain carrying wild-type β or β‘. A value of 4 indicates growth identical to wild type; 0 represents no plaque growth. In vitro Q activity is indicated by two values: the ratio of Q concentrations (WT/mutant) required for half saturation of readthrough activity (1/2sat); and the fraction of wild-type readthrough obtained at the highest Q concentration (RTmax). (B) Maps of RpoB and RpoC with conserved segments in blue. The regions displaying essentially identical tertiary folds between T. aquaticus RNAP and yeast Pol II are shown beneath in green (Ebright 2000). The rifampicin-binding site is shown (Jin and Gross 1988); the active center is defined by hydroxyl radical cleavage mediated by an Fe2+ ion chelated in the active site (Mustaev et al. 1997). Substitutions isolated with the Qλ reporter are at top (black), and those isolated with the Q82 reporter are below (gray); the dot color indicates the mutant class. DR, dispensable region.
Figure 2
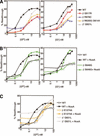
β-Galactosidase activity of the mutant RNAPs. (A) Activity of the Qλ reporter with substitutions isolated with the Qλ reporter. (B) Activity of the Q82 reporter with substitutions isolated with the Q82 reporter. (C) Activity of the Qλ reporter with substitutions isolated with the Q82 reporter. (D) Activity of the Q82 reporter with substitutions isolated with the Qλ reporter. Activities of the Q82 and Qλ reporters were identical with wild-type β or wild-type β‘, and only one curve is shown for simplicity. Q-protein synthesis was induced with arabinose. Synthesis of the mutant polymerase subunit was induced with 1% lactose.
Figure 3
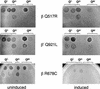
Phage spotting with mutant RNAPs. Tenfold serial dilutions of phage harboring different Q-proteins were spotted onto lawns of bacteria expressing the mutant RNAPs. Full results of the phage spotting are presented in Figure 1. Experimental plates contained 1% lactose to induce expression of the mutant RNAP subunit. Inducing expression of the wild-type subunits on pRL663 or pRL706 did not impair phage growth. Qλ phage are λ+ (left) and the clear mutant, λc17 (right); the Q21 phage is iλQ21; the Q82 phage are iλQ82 (left) and φ82 (right).
Figure 4
Gel analysis of in vitro transcription with purified mutant RNAPs. (A) A gel image of transcription with Qλ protein, at 0, 0.1, 1, 5, 10, 100, and 200 nM; all reactions contained 150 nM NusA. The positions of the abortive products (AP), +16/+17 (the σ70-dependent pause), terminated (T), and readthrough product (RT) are shown to the left. (B) Molar percent readthrough of terminator in experiment of A. (C) The gel image of abortive products (AP) and cleavage products (CP) made by β R678C RNAP. A single-round reaction was sampled at 0.25, 0.5, 1, 2, 3, 5, 8, and 12 min. Note that the absence of abortive products >8 nt in β R678C reveals a short-lived paused species at +11, +12, and +13.
Figure 5
Compilation of mutant RNAP activity in vitro. (A) Transcription is as in Figure 4 with a sample of RNAPs that were isolated against either Q-reporter. The right panel shows the level of Qλ-mediated antitermination supported by these substitutions; the left panel shows the Q82-mediated antitermination supported by these substitutions; all reactions contained 150 nM NusA. (B) Defect of β S646G in NusA-stimulated Q-mediated antitermination. (C) Termination defects of β E1274A and β‘ Q921L in the absence of NusA. The addition of NusA to transcription reactions (150 nM) eliminates much of the termination defect. Both mutant RNAPs are defective in response to Q, although the large backgrounds make the defect difficult to quantify.
Figure 6
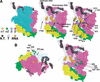
Structural model of the prokaryotic TEC, highlighting the position of substitutions. (A) A model of T. aquaticus TEC was used to map the substitutions onto the three-dimensional structure of RNAP (Korzheva et al. 2000). Equivalent positions in the T. aquaticus structure are highlighted and labeled with E. coli residue numbers. The left and center panels are equivalent except that the β subunit has been turned to a wire frame to expose the path of the nucleic acids through the enzyme. RNAP in this orientation is moving from left to right. The colors are as follows: α1 NTD, yellow; α2 NTD, green; β, cyan; β‘, violet; nontemplate strand, dark gray; template strand, light gray; RNA transcript, orange; β‘ f-helix, purple; β helix 673–687, dark blue; active-site Mg2+, dark green. Substitutions are labeled, space-filled, and colored as in Figure 1. Residue 1274 is hidden in this view, as indicated by the broken line. (B) The surface patch required for efficient Q activity, shown in two views of T. aquaticus RNAP. The left view is essentially down the secondary channel, and the right is as in A.
Figure 7
Substitutions within the active site and hybrid region of RNAP. A stereo view looking directly into the main channel of RNAP (RNAP is moving right to left in this view). Substitutions are colored and labeled equivalently to Figure 6 (none of the surface patch substitutions are seen in this view). The top image is essentially identical to that on the lower one, which contains the nucleic acids modeled into the complex with the template strand and RNA transcript shown in ball and stick (white and orange, respectively). The nontemplate strand was reduced to wire frame to clarify the image (gray). The colors are as follows: β, cyan; β‘, violet; both α NTDs, gray, for clarity only; β flap, dark blue, partially visible on extreme right side of both images; β‘ f-helix and rudder, purple; β helix from 673 to 687, dark blue.
Figure 7
Substitutions within the active site and hybrid region of RNAP. A stereo view looking directly into the main channel of RNAP (RNAP is moving right to left in this view). Substitutions are colored and labeled equivalently to Figure 6 (none of the surface patch substitutions are seen in this view). The top image is essentially identical to that on the lower one, which contains the nucleic acids modeled into the complex with the template strand and RNA transcript shown in ball and stick (white and orange, respectively). The nontemplate strand was reduced to wire frame to clarify the image (gray). The colors are as follows: β, cyan; β‘, violet; both α NTDs, gray, for clarity only; β flap, dark blue, partially visible on extreme right side of both images; β‘ f-helix and rudder, purple; β helix from 673 to 687, dark blue.
Similar articles
- The interaction surface of a bacterial transcription elongation factor required for complex formation with an antiterminator during transcription antitermination.
Mishra S, Mohan S, Godavarthi S, Sen R. Mishra S, et al. J Biol Chem. 2013 Sep 27;288(39):28089-103. doi: 10.1074/jbc.M113.472209. Epub 2013 Aug 2. J Biol Chem. 2013. PMID: 23913688 Free PMC article. - Structural basis of Q-dependent transcription antitermination.
Shi J, Gao X, Tian T, Yu Z, Gao B, Wen A, You L, Chang S, Zhang X, Zhang Y, Feng Y. Shi J, et al. Nat Commun. 2019 Jul 2;10(1):2925. doi: 10.1038/s41467-019-10958-8. Nat Commun. 2019. PMID: 31266960 Free PMC article. - Mechanisms of Bacterial Transcription Termination.
Roberts JW. Roberts JW. J Mol Biol. 2019 Sep 20;431(20):4030-4039. doi: 10.1016/j.jmb.2019.04.003. Epub 2019 Apr 9. J Mol Biol. 2019. PMID: 30978344 Review. - Hold on!: RNA polymerase interactions with the nascent RNA modulate transcription elongation and termination.
Grohmann D, Werner F. Grohmann D, et al. RNA Biol. 2010 May-Jun;7(3):310-5. doi: 10.4161/rna.7.3.11912. Epub 2010 May 26. RNA Biol. 2010. PMID: 20473037 Free PMC article. Review.
Cited by
- Insights into RNA polymerase catalysis and adaptive evolution gained from mutational analysis of a locus conferring rifampicin resistance.
Yurieva O, Nikiforov V Jr, Nikiforov V, O'Donnell M, Mustaev A. Yurieva O, et al. Nucleic Acids Res. 2017 Nov 2;45(19):11327-11340. doi: 10.1093/nar/gkx813. Nucleic Acids Res. 2017. PMID: 29036608 Free PMC article. - N protein from lambdoid phages transforms NusA into an antiterminator by modulating NusA-RNA polymerase flap domain interactions.
Mishra S, Sen R. Mishra S, et al. Nucleic Acids Res. 2015 Jul 13;43(12):5744-58. doi: 10.1093/nar/gkv479. Epub 2015 May 18. Nucleic Acids Res. 2015. PMID: 25990722 Free PMC article. - DNA binding regions of Q proteins of phages lambda and phi80.
Guo J, Roberts JW. Guo J, et al. J Bacteriol. 2004 Jun;186(11):3599-608. doi: 10.1128/JB.186.11.3599-3608.2004. J Bacteriol. 2004. PMID: 15150248 Free PMC article. - A processive riboantiterminator seeks a switch to make biofilms.
Artsimovitch I. Artsimovitch I. Mol Microbiol. 2010 May;76(3):535-9. doi: 10.1111/j.1365-2958.2010.07133.x. Epub 2010 Apr 8. Mol Microbiol. 2010. PMID: 20384681 Free PMC article. - Roles for the transcription elongation factor NusA in both DNA repair and damage tolerance pathways in Escherichia coli.
Cohen SE, Lewis CA, Mooney RA, Kohanski MA, Collins JJ, Landick R, Walker GC. Cohen SE, et al. Proc Natl Acad Sci U S A. 2010 Aug 31;107(35):15517-22. doi: 10.1073/pnas.1005203107. Epub 2010 Aug 9. Proc Natl Acad Sci U S A. 2010. PMID: 20696893 Free PMC article.
References
- Burgess RR, Jendrisak JJ. A procedure for the rapid, large-scale purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975;14:4634–4638. - PubMed
- Caldwell RC, Joyee GF. Randomization of genes by PCR mutagenesis. PCR Methods Applic. 1992;2:28–33. - PubMed
- Campbell EA, Korzheva N, Mustaev A, Murakami K, Nair S, Goldfarb A, Darst SA. Structural mechanism for rifampicin inhibition of bacterial RNA polymerase. Cell. 2001;104:901–912. - PubMed
- Cramer P, Bushnell DA, Fu J, Gnatt AL, Maier-Davis B, Thompson NE, Burgess RR, Edwards AM, David PR, Kornberg RD. Architecture of RNA polymerase II and implications for the transcription mechanism. Science. 2000;288:640–649. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 GM038660/GM/NIGMS NIH HHS/United States
- R37 GM021941/GM/NIGMS NIH HHS/United States
- GM21941/GM/NIGMS NIH HHS/United States
- GM38660/GM/NIGMS NIH HHS/United States
- R01 GM021941/GM/NIGMS NIH HHS/United States
- R01 GM038660/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources