Rous-Whipple Award Lecture. How tumors make bad blood vessels and stroma - PubMed (original) (raw)
Rous-Whipple Award Lecture. How tumors make bad blood vessels and stroma
Harold F Dvorak. Am J Pathol. 2003 Jun.
No abstract available
Figures
Figure 1.
a and b: Line 10 tumor cells 48 hours after transplant into the subcutaneous space of syngeneic strain 2 guinea pigs. Fibrin forms a water-trapping gel (F) that serves as a provisional stroma that separates tumor cells into discrete islands and that provides a favorable matrix for fibroblast (white arrows) and endothelial cell migration. c and d: Immunohistochemical demonstration of fibrin (brown staining) in guinea pig line 1 and human colorectal adenocarcinoma, respectively. e and f: Blood vessels (arrows) supplying line 10 guinea pig tumors are hyperpermeable to circulating macromolecular fluoresceinated dextran. g: Miles assay illustrating permeability to Evans blue-albumin complex at sites of intradermal injections of the following: (top, left to right) neutralizing anti-VEGF-A antibody, line 10 guinea pig tumor ascites fluid, mix of line 10 tumor ascites fluid and control IgG, mix of tumor ascites fluid and neutralizing anti-VEGF-A antibody. Bottom: line 1 tumor ascites fluid, mix of line 1 tumor ascites fluid and control IgG, and mix of line 1 tumor ascites fluid and neutralizing anti-VEGF-A antibody. h: Fibroblasts and blood vessels (black arrows) invade line 1 tumor fibrin gel, replacing it with fibrous connective tissue. i: Fibroblasts (arrows) migrate through fibrin gel (F) in vitro. j: Implanted fibrin gel (F) in subcutaneous space is replaced by ingrowth of fibroblasts and new blood vessels, creating granulation-like vascular connective tissue. Scale bars, 25 μm (b,i); 50 μm (a,c,d,h); 100 μm (e,f,j). e–g: Reprinted from Curr Top Microbiol Immunol 1999, 237:97–132 by copyright permission of Springer-Verlag GmbH & Co. KG; j reprinted from Lab Invest 1987, 57:673–686 by permission of Lippincott Williams & Wilkins, copyright The United States and Canadian Academy of Pathology, Inc.
Figure 2.
Angiogenic response to Ad-VEGF-A164 in the ears of nude mice at the indicated times and magnifications. (Reprinted from Cold Spring Harbor Symp Quant Biol 2002, 67:227–237 by copyright permission of Cold Spring Harbor Laboratory Press.).
Figure 3.
Electron micrographs 3 days after local Ad-VEGF-A164 injection and 20 minutes after i.v. injection of ferritin tracer. a: Hyperpermeable venule lined by endothelial cells of normal height, illustrating two prominent VVOs (collections of vesicles/vacuoles) that span endothelial cell cytoplasm from lumen (L) to abluminal basal lamina (BL). Note ferritin particles in vascular lumen, in VVO vesicles and vacuoles, and beneath BL (black dots, encircled). b: Mother vessel with greatly thinned endothelium spanned by four or fewer VVO vesicles/vacuoles. Note ferritin in these vesicles and vacuoles but tight junctions (arrows) remain closed and do not admit tracer. Bars, 200 nm. (Reprinted from Lab Invest 2000, 80:99–115 by permission of Lippincott Williams & Wilkins, copyright The United States and Canadian Academy of Pathology, Inc.)
Figure 4.
Mother vessels induced by Ad-VEGF-A164 (a–j), MOT tumor (k) and healing skin wound (l). a: Whole mount of colloidal carbon-perfused vascular bed. Mother vessels appear as enlarged segments of much smaller, normal venules. b: Confocal microscopic image of mother vessel stained for pericytes with an antibody to α-smooth-muscle actin. Note incomplete pericyte covering, especially over segments of greatest vessel enlargement. c: Developing mother vessels illustrating pericyte detachment (arrows). d: Mother vessel with detached pericyte (arrow) and activated endothelial cells whose large nuclei bulge into the vascular lumen, creating a highly irregular surface. e and f: Serpentine mother vessels with irregular luminal surface and endothelial cell bridging (f, arrow). g: Mother vessels (m) embedded in fibrin (arrows) provisional stroma. h: Loss of laminin staining (arrows) in developing mother vessels (m). i and j: Endothelial cell bridging (i, arrows) in mother vessels in skeletal muscle and ear skin, respectively. k and l: Mother vessels in mouse ovarian tumor and healing rat skin wound, respectively. Note bridging in k (arrows) and fibrin in l (lower, between cells). Scale bars, 20 μm (b–l); 100 μm (a). a and b: Reprinted from Cold Spring Harbor Symp Quant Biol 2002, 67:227–237 by copyright permission of Cold Spring Harbor Laboratory Press; c,e,f,h–j reprinted from Lab Invest 2000, 80:99–115 by permission of Lippincott Williams & Wilkins, copyright The United States and Canadian Academy of Pathology, Inc.
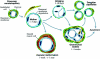
Figure 5.
Schematic diagram of mother vessel formation and evolution into daughter capillaries, vascular malformations and glomeruloid bodies. (Modified from Lab Invest 2000, 80:99–115 by permission of Lippincott Williams & Wilkins, copyright The United States and Canadian Academy of Pathology, Inc.)
Figure 6.
GB formation and devolution. a: Whole mount illustrates two GBs in ear of mouse perfused with colloidal carbon (white arrows), supplied by afferent and efferent mother vessels (m). b: 1-μm Epon section illustrates focal accumulation of large primitive cells (arrow) in a developing mother vessel (m). c: Immunohistochemistry demonstrates that such cells are CD31-positive. d: Primitive GB develops as focal nodule (between brackets) of proliferating cells in the wall of a mother vessel (m), extending both into the lumen and out into the extravascular connective tissue. Lip, adipocyte. e: Immunohistochemical staining for entactin demonstrates abundant basement membrane in a large GB (bracket) as well as in a mother vessel (m) and, conversely, very little in a lymphatic (L). f and g: Maturing GBs divide mother vessels into multiple, much smaller vascular channels (arrows). White arrows in f and h indicate apoptotic bodies. h and i: Devolving GBs reorganize into more normal-appearing microvessels (black arrows). Scale bars, 25 μm (c–f, h–i); 50 μm (a,b); 100 μm (g). (Reprinted from Am J Pathol 2001, 158:1145–1160 by copyright permission of the American Society for Investigative Pathology.)
Figure 7.
a and b: Vascular malformations induced by Ad-VEGF-A164 in ear skin at 35 and 131 days, respectively. L, giant lymphatics. c–f: Arteriogenesis following administration of Ad-VEGF-A164 in ear skin. Small arteries (c,d) enlarge by 6 to 7 days (e,f) accompanied by replication of endothelium and smooth muscle cells. Arrow (e), [3H]thymidine incorporated by a smooth-muscle cell. Scale bars, 50 μm (a,b); 15 μm (c–f). (Modified from Cold Spring Harbor Symp Quant Biol 2002, 67:227–237 by copyright permission of Cold Spring Harbor Laboratory Press.)
Figure 8.
a–d: Giant lymphatics in mouse ear skin at indicated times after injection of Ad-VEGF-A164. Lymphatics were perfused with colloidal carbon and viewed macroscopically. e–g: 1-μm Epon sections of giant lymphatics at indicated times after Ad-VEGF-A164. Lymphatics were injected with colloidal carbon (e). Scale bars, 50 μm. (Reprinted form J Exp Med 2002, 196:1497–1506 by copyright permission of The Rockefeller University Press.)
Similar articles
- Is there a role for nitric oxide in tumor angiogenesis?
García-Cardeña G, Folkman J. García-Cardeña G, et al. J Natl Cancer Inst. 1998 Apr 15;90(8):560-1. doi: 10.1093/jnci/90.8.560. J Natl Cancer Inst. 1998. PMID: 9580259 No abstract available. - [Significance of vascular endothelial growth factor--VEGF--in tumor angiogenesis. Therapeutic possibilities in solid tumors].
Werther K, Nielsen HJ. Werther K, et al. Ugeskr Laeger. 2000 Sep 11;162(37):4916-20. Ugeskr Laeger. 2000. PMID: 11002739 Danish. No abstract available. - VEGFs, angiopoietins, Ephrins and their receptors: putative targets for tumor therapy?
Marmé D. Marmé D. Ann Hematol. 2002;81 Suppl 2:S66. Ann Hematol. 2002. PMID: 12611080 No abstract available. - Endothelial receptor tyrosine kinases involved in angiogenesis.
Mustonen T, Alitalo K. Mustonen T, et al. J Cell Biol. 1995 May;129(4):895-8. doi: 10.1083/jcb.129.4.895. J Cell Biol. 1995. PMID: 7538139 Free PMC article. Review. No abstract available. - The biology of VEGF and its receptors.
Ferrara N, Gerber HP, LeCouter J. Ferrara N, et al. Nat Med. 2003 Jun;9(6):669-76. doi: 10.1038/nm0603-669. Nat Med. 2003. PMID: 12778165 Review.
Cited by
- Biomarker analyses in REGARD gastric/GEJ carcinoma patients treated with VEGFR2-targeted antibody ramucirumab.
Fuchs CS, Tabernero J, Tomášek J, Chau I, Melichar B, Safran H, Tehfe MA, Filip D, Topuzov E, Schlittler L, Udrea AA, Campbell W, Brincat S, Emig M, Melemed SA, Hozak RR, Ferry D, Caldwell CW, Ajani JA. Fuchs CS, et al. Br J Cancer. 2016 Oct 11;115(8):974-982. doi: 10.1038/bjc.2016.293. Epub 2016 Sep 13. Br J Cancer. 2016. PMID: 27623234 Free PMC article. - Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin.
Phung TL, Ziv K, Dabydeen D, Eyiah-Mensah G, Riveros M, Perruzzi C, Sun J, Monahan-Earley RA, Shiojima I, Nagy JA, Lin MI, Walsh K, Dvorak AM, Briscoe DM, Neeman M, Sessa WC, Dvorak HF, Benjamin LE. Phung TL, et al. Cancer Cell. 2006 Aug;10(2):159-70. doi: 10.1016/j.ccr.2006.07.003. Cancer Cell. 2006. PMID: 16904613 Free PMC article. - Identification of mRNAs that continue to associate with polysomes during hypoxia.
Thomas JD, Johannes GJ. Thomas JD, et al. RNA. 2007 Jul;13(7):1116-31. doi: 10.1261/rna.534807. Epub 2007 May 8. RNA. 2007. PMID: 17488873 Free PMC article. - Role of prostaglandin E2-dependent angiogenic switch in cyclooxygenase 2-induced breast cancer progression.
Chang SH, Liu CH, Conway R, Han DK, Nithipatikom K, Trifan OC, Lane TF, Hla T. Chang SH, et al. Proc Natl Acad Sci U S A. 2004 Jan 13;101(2):591-6. doi: 10.1073/pnas.2535911100. Epub 2003 Dec 19. Proc Natl Acad Sci U S A. 2004. PMID: 14688410 Free PMC article. - Endothelial miR-30c suppresses tumor growth via inhibition of TGF-β-induced Serpine1.
McCann JV, Xiao L, Kim DJ, Khan OF, Kowalski PS, Anderson DG, Pecot CV, Azam SH, Parker JS, Tsai YS, Wolberg AS, Turner SD, Tatsumi K, Mackman N, Dudley AC. McCann JV, et al. J Clin Invest. 2019 Mar 11;129(4):1654-1670. doi: 10.1172/JCI123106. eCollection 2019 Mar 11. J Clin Invest. 2019. PMID: 30855280 Free PMC article.
References
- Dvorak HF: Tumors: wounds that do not heal: similarities between tumor stroma generation and wound healing. N Engl J Med 1986, 315:1650-1659 - PubMed
- Dvorak H, Nagy J, Feng D, Dvorak A: Tumor architecture and targeted delivery. Abrams P Fritzberg A eds. Radioimmunotherapy of Cancer. 2000:pp 107-135 Marcel Dekker, Inc., New York
- Folkman J: Tumor angiogenesis. Adv Cancer Res 1985, 43:175-203 - PubMed
- Brown LF, Detmar M, Claffey K, Nagy JA, Feng D, Dvorak AM, Dvorak HF: Vascular permeability factor/vascular endothelial growth factor: a multifunctional angiogenic cytokine. Exs 1997, 79:233-269 - PubMed
- Dvorak HF, Nagy JA, Feng D, Brown LF, Dvorak AM: Vascular permeability factor/vascular endothelial growth factor and the significance of microvascular hyperpermeability in angiogenesis. Curr Top Microbiol Immunol 1999, 237:97-132 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA92644/CA/NCI NIH HHS/United States
- R01 CA050453/CA/NCI NIH HHS/United States
- P01 CA092644/CA/NCI NIH HHS/United States
- HL-59316/HL/NHLBI NIH HHS/United States
- CA-50453/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials