Tumor lymphangiogenesis: a novel prognostic indicator for cutaneous melanoma metastasis and survival - PubMed (original) (raw)
Tumor lymphangiogenesis: a novel prognostic indicator for cutaneous melanoma metastasis and survival
Soheil S Dadras et al. Am J Pathol. 2003 Jun.
Abstract
Malignant melanomas of the skin are distinguished by their propensity for early metastatic spread via lymphatic vessels to regional lymph nodes, and lymph node metastasis is a major determinant for the staging and clinical management of melanoma. However, the importance of tumor-induced lymphangiogenesis for lymphatic melanoma spread has remained unclear. We investigated whether tumor lymphangiogenesis occurs in human malignant melanomas of the skin and whether the extent of tumor lymphangiogenesis may be related to the risk for lymph node metastasis and to patient survival, using double immunostains for the novel lymphatic endothelial marker LYVE-1 and for the panvascular marker CD31. Tumor samples were obtained from clinically and histologically closely matched cases of primary melanomas with early lymph node metastasis (n = 18) and from nonmetastatic melanomas (n = 19). Hot spots of proliferating intratumoral and peritumoral lymphatic vessels were detected in a large number of melanomas. The incidence of intratumoral lymphatics was significantly higher in metastatic melanomas and correlated with poor disease-free survival. Metastatic melanomas had significantly more and larger tumor-associated lymphatic vessels, and a relative lymphatic vessel area of >1.5% was significantly associated with poor disease-free and overall survival. In contrast, no differences in the density of tumor-associated blood vessels were found. Vascular endothelial growth factor and vascular endothelial growth factor-C expression was equally detected in a minority of cases in both groups. Our results reveal tumor lymphangiogenesis as a novel prognostic indicator for the risk of lymph node metastasis in cutaneous melanoma.
Figures
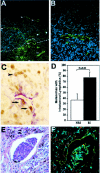
Figure 1.
Detection of intratumoral and peritumoral lymphatic vessels in cutaneous malignant melanomas. A: Histology of a thick melanoma (4.5 mm) shows compact masses of frequently pigmented tumor cells. B: Immunofluorescent stain of a serial section of the same tumor for the lymphatic marker LYVE-1 (green) and the panvascular marker CD31 (red) reveals prominent hotspots of high lymphatic vessel density (hotspots are circled by solid line). In contrast, blood vessels are homogeneously distributed throughout the tumor. C: Thin-walled, LYVE-1-positive intratumoral lymphatic vessels with open lumina. D: Histology of a thin melanoma (≤1.5 mm) with dilated peritumoral lymphatics (arrows). E: Immunofluorescent stain of a serial section for LYVE-1 (green) and CD31 (red) reveals lymphatic vessels within (arrowheads) and surrounding (arrows) the tumor border (dotted line). F: LYVE-1-positive intratumoral lymphatic vessels with open lumina. The adjacent blood vessels (asterisks) are LYVE-1-negative. Cell nuclei are counterstained (blue) with Hoechst (C and F). Original magnifications: ×100 (A, B, D, and E); ×400 (C and F).
Figure 2.
Higher frequency of intratumoral lymphangiogenesis in metastatic melanomas. A: Immunofluorescent stain for LYVE-1 (green) depicts thick-walled peritumoral (arrows) and thin-walled intratumoral lymphatics (arrowheads) in a thin melanoma (1.05 mm). The tumor border is indicated by a dotted line. Blood vessels (asterisks) are negative for LYVE-1. B: Higher magnification of intratumoral lymphatics reveals thin-walled, basket-like morphology. C: Double immunostain for LYVE-1 (red) and PCNA (brown) reveals an intratumoral lymphatic vessel with proliferating lymphatic endothelial cells (arrows) and adjacent melanoma cells (arrowhead). D: Significantly increased frequency of detectable intratumoral lymphatic vessels in metastatic melanomas (M; n = 18), as compared with nonmetastatic (NM; n = 19) tumors (mean ± SEM, chi-square test, P = 0.01). E: Detection of melanin-containing tumor cells within an intratumoral lymphatic vessel. H&E stain. F: Immunofluorescent stain of a serial section for LYVE-1 (green) and CD31 (red) confirms that tumor cells are located within a lymphatic vessel. Cell nuclei are counterstained blue with Hoechst (B and F). Original magnifications: ×200 (A); ×400 (B, C, E, F).
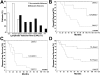
Figure 3.
Computer-assisted image analysis of tumor-associated blood vessels and peritumoral lymphatic vessels in nonmetastatic versus metastatic melanomas. A–C: Comparable blood vascular density, average blood vessel size, and relative area occupied by blood vessels in metastatic (M; n = 18) and nonmetastatic (NM; n = 19) malignant melanomas. D–F: Significant increase of lymphatic vascular density, average lymphatic vessel size, and relative area occupied by lymphatics in metastatic malignant melanomas, as compared with nonmetastatic melanomas. Mean ± SEM.
Figure 4.
VEGF-C mRNA expression in cutaneous melanomas. A: Bright-field microscopy shows a dermal melanoma nodule. B: Dark-field microscopy reveals increased signal (white grains) in the tumor area but not the dermis. C: Bright-field microscopy shows increased black grains under a higher magnification from the rectangle in A. D: VEGF-C mRNA expression by peritumoral stromal cells. Arrows indicate areas of strong hybridization signals. Original magnifications: ×200 (A and B); ×600 (C and D).
Figure 5.
VEGF-C protein expression in cutaneous melanomas. A: Immunoperoxidase staining for VEGF-C (red) demonstrates focal expression in the cytoplasm of melanoma cells in one tumor nest but not in the adjacent nests (asterisks). B: Serial section negative control with omission of secondary antibody. C: Immunoperoxidase staining for VEGF-C (red) demonstrates VEGF-C expression by peritumoral fibroblasts (rectangle) at the invasive edge of a metastatic melanoma (top left) in the reticular dermis. D: H&E stain of the invasive tumor edge of a metastasizing cutaneous melanoma reveals mononuclear inflammation (asterisks) near dilated lymphatic vessel (arrow). E: Immunoperoxidase staining of a serial section for LYVE-1 (red) decorates dilated peritumoral lymphatics (arrow) and adjacent mononuclear infiltrate. Blood vessels are LYVE-1-negative (arrowheads). F: Immunoperoxidase staining for VEGF-C (red) stains the cytoplasm of peritumoral mononuclear cells in a granular pattern. Original magnifications: ×200 (A–D); ×400 (E); and ×600 (F).
Figure 6.
Kaplan-Meier analyses of overall and disease-free survival as a function of tumor lymphangiogenesis. A: The distribution of lymphatic vascular area (LVA) in the patient population indicates an overlap in the >1.0 to ≤1.5 range between the nonmetastatic and metastatic melanoma groups. B, C: Overall and disease-free survival curves were stratified by low (≤1.0%), medium (1.0 to ≤1.5%), or high (>1.5%) level of tumor lymphangiogenesis. Statistically significant correlation between the extent of tumor-associated lymphangiogenesis, expressed as percentage of area covered by lymphatic vessels, and reduced disease-free survival and overall survival are noted. D: The disease-free survival analysis revealed that the detection of intratumoral lymphatics was significantly associated with a poorer disease-free survival.
Similar articles
- Lymphangiogenesis and its relationship with lymphatic metastasis and prognosis in malignant melanoma.
Liu B, Ma J, Wang X, Su F, Li X, Yang S, Ma W, Zhang Y. Liu B, et al. Anat Rec (Hoboken). 2008 Oct;291(10):1227-35. doi: 10.1002/ar.20736. Anat Rec (Hoboken). 2008. PMID: 18561194 - Significance of Vascular Endothelial Growth Factor (VEGF)-C and VEGF-D in the Progression of Cutaneous Melanoma.
Špirić Z, Eri Ž, Erić M. Špirić Z, et al. Int J Surg Pathol. 2015 Dec;23(8):629-37. doi: 10.1177/1066896915583694. Epub 2015 Apr 24. Int J Surg Pathol. 2015. PMID: 25911567 - Expression pattern of the lymphatic and vascular markers VEGFR-3 and CD31 does not predict regional lymph node metastasis in cutaneous melanoma.
Wobser M, Siedel C, Schrama D, Bröcker EB, Becker JC, Vetter-Kauczok CS. Wobser M, et al. Arch Dermatol Res. 2006 Feb;297(8):352-7. doi: 10.1007/s00403-005-0633-1. Epub 2006 Jan 3. Arch Dermatol Res. 2006. PMID: 16395613 - Angiogenesis and lymphangiogenesis of skin cancers.
Dadras SS, Detmar M. Dadras SS, et al. Hematol Oncol Clin North Am. 2004 Oct;18(5):1059-70, viii. doi: 10.1016/j.hoc.2004.06.009. Hematol Oncol Clin North Am. 2004. PMID: 15474335 Review. - Tumor lymphangiogenesis and melanoma metastasis.
Rinderknecht M, Detmar M. Rinderknecht M, et al. J Cell Physiol. 2008 Aug;216(2):347-54. doi: 10.1002/jcp.21494. J Cell Physiol. 2008. PMID: 18481261 Review.
Cited by
- Lymphatic system regulation of anti-cancer immunity and metastasis.
Lei PJ, Fraser C, Jones D, Ubellacker JM, Padera TP. Lei PJ, et al. Front Immunol. 2024 Aug 15;15:1449291. doi: 10.3389/fimmu.2024.1449291. eCollection 2024. Front Immunol. 2024. PMID: 39211044 Free PMC article. Review. - Cancer metastasis through the lymphatic versus blood vessels.
Leong SP, Witte MH. Leong SP, et al. Clin Exp Metastasis. 2024 Aug;41(4):387-402. doi: 10.1007/s10585-024-10288-0. Epub 2024 Jun 28. Clin Exp Metastasis. 2024. PMID: 38940900 Free PMC article. Review. - Angiogenesis Still Plays a Crucial Role in Human Melanoma Progression.
Cazzato G, Ingravallo G, Ribatti D. Cazzato G, et al. Cancers (Basel). 2024 May 8;16(10):1794. doi: 10.3390/cancers16101794. Cancers (Basel). 2024. PMID: 38791873 Free PMC article. Review. - Anti-lymphangiogenesis for boosting drug accumulation in tumors.
Wang C, Xu J, Cheng X, Sun G, Li F, Nie G, Zhang Y. Wang C, et al. Signal Transduct Target Ther. 2024 Apr 15;9(1):89. doi: 10.1038/s41392-024-01794-4. Signal Transduct Target Ther. 2024. PMID: 38616190 Free PMC article. - Quantitative multiplex immunohistochemistry reveals inter-patient lymphovascular and immune heterogeneity in primary cutaneous melanoma.
Femel J, Hill C, Illa Bochaca I, Booth JL, Asnaashari TG, Steele MM, Moshiri AS, Do H, Zhong J, Osman I, Leachman SA, Tsujikawa T, White KP, Chang YH, Lund AW. Femel J, et al. Front Immunol. 2024 Feb 1;15:1328602. doi: 10.3389/fimmu.2024.1328602. eCollection 2024. Front Immunol. 2024. PMID: 38361951 Free PMC article.
References
- Landis SH, Murray T, Bolden S, Wingo PA: Cancer statistics, 1998. CA Cancer J Clin 1998, 48:6-29 - PubMed
- Clark WH, Jr, From L, Bernardino EA, Mihm MC: The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res 1969, 29:705-727 - PubMed
- Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, Fleming ID, Gershenwald JE, Houghton A, Jr, Kirkwood JM, McMasters KM, Mihm MF, Morton DL, Reintgen DS, Ross MI, Sober A, Thompson JA, Thompson JF: Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol 2001, 19:3635-3648 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA86410/CA/NCI NIH HHS/United States
- 5T32CA09216/CA/NCI NIH HHS/United States
- R01 CA069184/CA/NCI NIH HHS/United States
- P01 CA092644/CA/NCI NIH HHS/United States
- CA92644/CA/NCI NIH HHS/United States
- T32 CA009216/CA/NCI NIH HHS/United States
- CA69184/CA/NCI NIH HHS/United States
- R01 CA086410/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous