Nitric oxide synthase 3-dependent vascular remodeling and circulatory dysfunction in cirrhosis - PubMed (original) (raw)
Nitric oxide synthase 3-dependent vascular remodeling and circulatory dysfunction in cirrhosis
Guillermo Fernández-Varo et al. Am J Pathol. 2003 Jun.
Abstract
Vascular remodeling is an active process that consists in important modifications in the vessel wall. Endothelium-derived nitric oxide (NO) plays a major role in this phenomenon. We assessed wall thickness (WT), total wall area (TWA), lumen diameter, and total nuclei number/cross-section (TN) in cirrhotic rats with ascites and in control rats. A second group of cirrhotic rats received the NO synthesis inhibitor, L-NAME, or vehicle daily for 11 weeks and systemic hemodynamics, arterial compliance, aortic NO synthase 3 (NOS3) protein expression, and vascular morphology were analyzed. Cirrhotic vessels showed a significant reduction in WT, TWA, and TN as compared to control vessels. Long-term inhibition of NOS activity in cirrhotic rats resulted in a significant increase in WT, TWA, and TN as compared to cirrhotic rats receiving vehicle. NOS3 protein abundance was higher in aortic vessels of nontreated cirrhotic animals than in controls. This difference was abolished by chronic treatment with L-NAME. NOS inhibition in cirrhotic rats resulted in higher arterial pressure and peripheral resistance and lower arterial compliance than cirrhotic rats receiving vehicle. Therefore, vascular remodeling in cirrhosis with ascites is a generalized process with significant functional consequences that can be negatively modulated by long-term inhibition of NOS activity.
Figures
Figure 1.
Photomicrographs of representative cross sections of thoracic and abdominal aorta, and mesenteric and renal arteries from a control rat (A, C, E, and G, respectively) and a cirrhotic rat with ascites (B, D, F, and H, respectively). Note the marked reduction in WT and the diminution in the number of nuclei in the cirrhotic vessel (H&E staining; original magnifications, ×200).
Figure 2.
WT, LD, TWA, and WT/LD ratio in control rats and in cirrhotic rats with ascites. THORAC, thoracic aorta; ABDOM, abdominal aorta; MES, mesenteric artery; REN, renal artery. Data are mean ± SE; n = 6 rats per group.
Figure 3.
WT, LD, TWA, and WT/LD ratio in cirrhotic rats receiving vehicle or chronically treated with L-NAME (0.5 mg/kg/day). THORAC, thoracic aorta; ABDOM, abdominal aorta; MES, mesenteric artery; REN, renal artery. Data are mean ± SE; n = 7 rats per group.
Figure 4.
Aortic cGMP concentration in control and cirrhotic rats receiving vehicle or chronically treated with L-NAME (0.5 mg/kg/day). Thoracic aortas were individually homogenized and the concentration of cGMP was measured in the acetylated extracts by radioimmunoassay. Eight cirrhotic and 12 control rats were studied, half of the animals in each group being treated with L-NAME. Data are mean ± SE.
Figure 5.
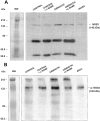
Representative Western blot of total NOS3 (A) and phospho-NOS3 (p-NOS3) (B) protein in thoracic aorta of control and cirrhotic rats receiving vehicle or chronically treated with L-NAME (0.5 mg/kg/day). Protein extracts were prepared as described in Material and Methods and 40 μg (total NOS3) or 80 μg (p-NOS3) of protein were loaded per lane. Ten μg of human vein endothelial cell (HUVEC) lysate or stimulated bovine aortic endothelial cells (BAEC) were used as a positive control.
Similar articles
- The role of nitric oxide in the pathogenesis of systemic and splanchnic vasodilation in cirrhotic rats before and after the onset of ascites.
Angeli P, Fernández-Varo G, Dalla Libera V, Fasolato S, Galioto A, Arroyo V, Sticca A, Guarda S, Gatta A, Jiménez W. Angeli P, et al. Liver Int. 2005 Apr;25(2):429-37. doi: 10.1111/j.1478-3231.2005.01092.x. Liver Int. 2005. PMID: 15780069 - Increased nitric oxide synthase expression in arterial vessels of cirrhotic rats with ascites.
Morales-Ruiz M, Jiménez W, Pérez-Sala D, Ros J, Leivas A, Lamas S, Rivera F, Arroyo V. Morales-Ruiz M, et al. Hepatology. 1996 Dec;24(6):1481-6. doi: 10.1053/jhep.1996.v24.pm0008938184. Hepatology. 1996. PMID: 8938184 - Chronic inhibition of nitric-oxide synthase potentiates endothelium-dependent contractions in the rat aorta by augmenting the expression of cyclooxygenase-2.
Qu C, Leung SW, Vanhoutte PM, Man RY. Qu C, et al. J Pharmacol Exp Ther. 2010 Aug;334(2):373-80. doi: 10.1124/jpet.110.167098. Epub 2010 May 5. J Pharmacol Exp Ther. 2010. PMID: 20444882 - Nitric oxide synthase (NOS) inhibition for one week improves renal sodium and water excretion in cirrhotic rats with ascites.
Martin PY, Ohara M, Gines P, Xu DL, St John J, Niederberger M, Schrier RW. Martin PY, et al. J Clin Invest. 1998 Jan 1;101(1):235-42. doi: 10.1172/JCI626. J Clin Invest. 1998. PMID: 9421486 Free PMC article. - Endothelium-dependent vascular hyporesponsiveness without detection of nitric oxide synthase induction in aortas of cirrhotic rats.
Weigert AL, Martin PY, Niederberger M, Higa EM, McMurtry IF, Gines P, Schrier RW. Weigert AL, et al. Hepatology. 1995 Dec;22(6):1856-62. Hepatology. 1995. PMID: 7489998
Cited by
- Monocyte-endothelial cell interactions in vascular and tissue remodeling.
Medrano-Bosch M, Simón-Codina B, Jiménez W, Edelman ER, Melgar-Lesmes P. Medrano-Bosch M, et al. Front Immunol. 2023 Jul 7;14:1196033. doi: 10.3389/fimmu.2023.1196033. eCollection 2023. Front Immunol. 2023. PMID: 37483594 Free PMC article. Review. - COX-2/sEH Dual Inhibitor PTUPB Alleviates CCl 4 -Induced Liver Fibrosis and Portal Hypertension.
Zhao Z, Zhang C, Lin J, Zheng L, Li H, Qi X, Huo H, Lou X, Hammock BD, Hwang SH, Bao Y, Luo M. Zhao Z, et al. Front Med (Lausanne). 2021 Dec 23;8:761517. doi: 10.3389/fmed.2021.761517. eCollection 2021. Front Med (Lausanne). 2021. PMID: 35004731 Free PMC article. - Biology of portal hypertension.
McConnell M, Iwakiri Y. McConnell M, et al. Hepatol Int. 2018 Feb;12(Suppl 1):11-23. doi: 10.1007/s12072-017-9826-x. Epub 2017 Oct 26. Hepatol Int. 2018. PMID: 29075990 Free PMC article. Review. - Restructuring of the vascular bed in response to hemodynamic disturbances in portal hypertension.
Garbuzenko DV, Arefyev NO, Belov DV. Garbuzenko DV, et al. World J Hepatol. 2016 Dec 28;8(36):1602-1609. doi: 10.4254/wjh.v8.i36.1602. World J Hepatol. 2016. PMID: 28083082 Free PMC article. Review. - Vascular pathobiology in chronic liver disease and cirrhosis - current status and future directions.
Iwakiri Y, Shah V, Rockey DC. Iwakiri Y, et al. J Hepatol. 2014 Oct;61(4):912-24. doi: 10.1016/j.jhep.2014.05.047. Epub 2014 Jun 6. J Hepatol. 2014. PMID: 24911462 Free PMC article. Review.
References
- Gibbons GH, Dzau VJ: The emerging concept of vascular remodeling. N Engl J Med 1994, 330:1431-1438 - PubMed
- Mulvany MJ: Vascular remodeling of resistance vessels: can we define this? Cardiovasc Res 1999, 41:9-13 - PubMed
- Dzau VJ, Horiuchi M: Vascular remodeling: the emerging paradigm of programmed cell death (apoptosis). Chest 1998, 114:91S-99S - PubMed
- Arroyo V, Jiménez W: Renal and circulatory dysfunction in cirrhosis. Lights and shadows in an important clinical problem. J Hepatol 2000, 32(Suppl 1):157-170 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources