Experimental diabetes causes breakdown of the blood-retina barrier by a mechanism involving tyrosine nitration and increases in expression of vascular endothelial growth factor and urokinase plasminogen activator receptor - PubMed (original) (raw)
Experimental diabetes causes breakdown of the blood-retina barrier by a mechanism involving tyrosine nitration and increases in expression of vascular endothelial growth factor and urokinase plasminogen activator receptor
Azza B El-Remessy et al. Am J Pathol. 2003 Jun.
Abstract
The purpose of these experiments was to determine the specific role of reactive oxygen species (ROS) in the blood-retinal barrier (BRB) breakdown that characterizes the early stages of vascular dysfunction in diabetes. Based on our data showing that high glucose increases nitric oxide, superoxide, and nitrotyrosine formation in retinal endothelial cells, we hypothesized that excess formation of ROS causes BRB breakdown in diabetes. Because ROS are known to induce increases in expression of the well-known endothelial mitogen and permeability factor vascular endothelial growth factor (VEGF) we also examined their influence on the expression of VEGF and its downstream target urokinase plasminogen activator receptor (uPAR). After 2 weeks of streptozotocin-induced diabetes, analysis of albumin leakage confirmed a prominent breakdown of the BRB. This permeability defect was correlated with significant increases in the formation of nitric oxide, lipid peroxides, and the peroxynitrite biomarker nitrotyrosine as well as with increases in the expression of VEGF and uPAR. Treatment with a nitric oxide synthase inhibitor (N-omega-nitro-L-arginine methyl ester, 50 mg/kg/day) or peroxynitrite scavenger (uric acid, 160 mg/kg/day) blocked the breakdown in the BRB and prevented the increases in formation of lipid peroxides and tyrosine nitration as well as the increases in expression of VEGF and uPAR. Taken together, these data indicate that early diabetes causes breakdown of the BRB by a mechanism involving the action of reactive nitrogen species in promoting expression of VEGF and uPAR.
Figures
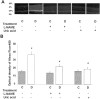
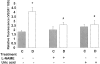
Figure 1.
Permeability as determined by Alexa-Fluor 488-BSA in STZ-induced diabetic and control rat retina. Diabetic and control rats were injected intravenously with Alexa-Fluor 488-BSA (100 mg/kg each). A: Representative images showing the fluorescence distribution in different retinal layers; the nerve fiber layer (NFL), inner plexiform layer (IPL), outer plexiform layer (OPL), and the inner segment layer (ISL). B: Morphometric analysis of fluorescence intensity in serial sections of rat eyes shows that diabetic rats had a twofold increase in fluorescence compared with controls. Treatment of diabetic rats with L-NAME (50 mg/kg/day) or with uric acid (160 mg/kg/day) blocked the permeability increase. Data shown is the mean ± SEM of six animals in each group. C, control; D, diabetic; *, P < 0.01 versus untreated control; #, P < 0.05 versus untreated diabetic. Original magnification, ×200 (A).
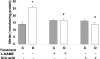
Figure 2.
Levels of nitrate/nitrite in STZ-induced diabetic and control rat retinas as determined by the Greiss reaction assay. Diabetic retinas had significant increases in formation of nitrate/nitrite. Treatment of diabetic rats with L-NAME (50 mg/kg/day) or with uric acid (160 mg/kg/day) blocked the increased nitrate/nitrite formation. Data shown is the mean ± SEM of six animals in the treated groups and nine animals in untreated groups. C, control; D, diabetic; *, P < 0.01 versus untreated control; #, P < 0.05 versus untreated diabetic.
Figure 3.
Levels of lipid peroxides in STZ-induced diabetic and control rat retinas as determined by the amount of thiobarbituric acid reactivity with malondialdehyde formed during acid hydrolysis of the lipid peroxide. Diabetic retinas had significant increases in formation of lipid peroxides. Treatment of diabetic rats with L-NAME (50 mg/kg/day) or with uric acid (160 mg/kg/day) blocked the increased lipid peroxidation. Data shown is the mean ± SEM of six animals in the treated groups and nine animals in untreated groups. C, control; D, diabetic; *, P < 0.01 versus untreated control; #, P < 0.05 versus untreated diabetic.
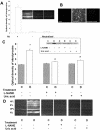
Figure 4.
Measures of nitrotyrosine formation in peroxynitrite-treated endothelial cells and STZ-induced diabetic and control rats. A: Control experiments demonstrating the specificity of the nitrotyrosine antibody. The signal was absent in the absence of the primary antibody or in the presence of neutralized primary antibody. B: Immunocytochemistry of nitrotyrosine in cultured bovine endothelial cells. Peroxynitrite (100 μmol/L) increases nitrotyrosine formation and uric acid (1 mmol/L) blocked the increased nitrotyrosine formation. C: Slot-blot analysis of nitrotyrosine immunoreactivity. Window shows a representative slot blot. Diabetes increases nitrotyrosine formation by 1.8-fold. Treatment of diabetic rats with L-NAME (50 mg/kg/day) or with uric acid (160 mg/kg/day) blocked the increased nitrotyrosine formation. Data shown is the mean ± SEM of six animals in the treated groups and nine animals in untreated groups. D: Immunohistochemistry of nitrotyrosine in diabetic and control rats, note strong immunoreactivity in the vascularized layers [nerve fiber layer (NFL), inner plexiform layer (IPL), and outer plexiform layer (OPL)]. C, control; D, diabetic; *, P < 0.01 versus untreated control; #, P < 0.05 versus untreated diabetic. Original magnification, ×200 (D).
Figure 5.
Analysis of NOS protein expression and activity in STZ-induced diabetic and control rat retinas. Data shown is the mean ± SEM of six animals in the treated groups and nine animals in untreated groups. A: Analysis of maximal NOS activity by conversion of [3H]-
l-
arginine to [3H]-
l-
citrulline in tissue homogenates shows significant increases in NOS activity in diabetic retinas. Treatment of diabetic rats with L-NAME (50 mg/kg/day) or with uric acid (160 mg/kg/day) blocked the increased NOS activity. C, control; D, diabetic; *, P < 0.01 versus untreated control; #, P < 0.05 versus untreated diabetic. B: Western blot analysis shows a significant increase in eNOS expression in diabetic retinas, which is blunted by treatment with L-NAME (50 mg/kg/day) or uric acid (160 mg/kg/day). C: Western blot analysis shows a significant increase in nNOS expression in diabetic retinas, which is blocked by treatment with L-NAME (50 mg/kg/day) or uric acid (160 mg/kg/day).
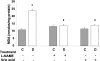
Figure 6.
Expression of VEGF mRNA in diabetic and control rat retina as determined by real-time PCR. STZ-induced diabetic retinas had significant increases in VEGF mRNA expression. This increase was totally blocked by treatment with L-NAME (50 mg/kg/day) or uric acid (160 mg/kg/day). Data shown is the mean ± SEM of three animals in each group. C, control; D, diabetic; *, P < 0.01 versus untreated control; #, P < 0.05 versus untreated diabetic.
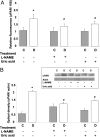
Figure 7.
Expression of uPAR mRNA and protein levels in STZ-induced diabetic and control rat retina as determined by real-time PCR and Western blot analysis. A: Diabetic retinas had significant increases in uPAR mRNA expression, which was inhibited by treatment with L-NAME (50 mg/kg/day) or uric acid (160 mg/kg/day). Data shown is the mean ± SEM of three animals in each group. B: Diabetic retinas had significant increases in uPAR protein expression, which was inhibited by treatment with L-NAME (50 mg/kg/day) or uric acid (160 mg/kg/day). Data shown is the mean ± SEM of six animals in each group. C, control; D, diabetic; *, P < 0.01 versus untreated control; #, P < 0.05 versus untreated diabetic.
Similar articles
- Retinal vascular endothelial growth factor induces intercellular adhesion molecule-1 and endothelial nitric oxide synthase expression and initiates early diabetic retinal leukocyte adhesion in vivo.
Joussen AM, Poulaki V, Qin W, Kirchhof B, Mitsiades N, Wiegand SJ, Rudge J, Yancopoulos GD, Adamis AP. Joussen AM, et al. Am J Pathol. 2002 Feb;160(2):501-9. doi: 10.1016/S0002-9440(10)64869-9. Am J Pathol. 2002. PMID: 11839570 Free PMC article. - Diabetes-induced superoxide anion and breakdown of the blood-retinal barrier: role of the VEGF/uPAR pathway.
El-Remessy AB, Franklin T, Ghaley N, Yang J, Brands MW, Caldwell RB, Behzadian MA. El-Remessy AB, et al. PLoS One. 2013 Aug 7;8(8):e71868. doi: 10.1371/journal.pone.0071868. eCollection 2013. PLoS One. 2013. PMID: 23951261 Free PMC article. - Significance of nitric oxide and peroxynitrite in permeability changes of the retinal microvascular endothelial cell monolayer induced by vascular endothelial growth factor.
Marumo T, Noll T, Schini-Kerth VB, Harley EA, Duhault J, Piper HM, Busse R. Marumo T, et al. J Vasc Res. 1999 Nov-Dec;36(6):510-5. doi: 10.1159/000025694. J Vasc Res. 1999. PMID: 10629427 - The relation between expression of vascular endothelial growth factor and breakdown of the blood-retinal barrier in diabetic rat retinas.
Murata T, Nakagawa K, Khalil A, Ishibashi T, Inomata H, Sueishi K. Murata T, et al. Lab Invest. 1996 Apr;74(4):819-25. Lab Invest. 1996. PMID: 8606491 - Tyrosine nitration as mediator of cell death.
Franco MC, Estévez AG. Franco MC, et al. Cell Mol Life Sci. 2014 Oct;71(20):3939-50. doi: 10.1007/s00018-014-1662-8. Epub 2014 Jun 20. Cell Mol Life Sci. 2014. PMID: 24947321 Free PMC article. Review.
Cited by
- Peroxynitrite mediates glomerular lesion of diabetic rat via JAK/STAT signaling pathway.
Wang H, Li Y, Liu H, Liu S, Liu Q, Wang XM, Shi Y, Duan H. Wang H, et al. J Endocrinol Invest. 2009 Nov;32(10):844-51. doi: 10.1007/BF03345756. Epub 2009 Jul 28. J Endocrinol Invest. 2009. PMID: 19636222 - Nanomedicines for back of the eye drug delivery, gene delivery, and imaging.
Kompella UB, Amrite AC, Pacha Ravi R, Durazo SA. Kompella UB, et al. Prog Retin Eye Res. 2013 Sep;36:172-98. doi: 10.1016/j.preteyeres.2013.04.001. Epub 2013 Apr 17. Prog Retin Eye Res. 2013. PMID: 23603534 Free PMC article. Review. - Immunogold study of altered expression of some interendothelial junctional molecules in the brain blood microvessels of diabetic scrapie-infected mice.
Vorbrodt AW, Dobrogowska DH, Tarnawski M, Meeker HC, Carp RI. Vorbrodt AW, et al. J Mol Histol. 2006 Jan;37(1-2):27-35. doi: 10.1007/s10735-006-9026-9. Epub 2006 Aug 22. J Mol Histol. 2006. PMID: 16724250 - Diabetic retinopathy: loss of neuroretinal adaptation to the diabetic metabolic environment.
Abcouwer SF, Gardner TW. Abcouwer SF, et al. Ann N Y Acad Sci. 2014 Apr;1311:174-90. doi: 10.1111/nyas.12412. Epub 2014 Mar 27. Ann N Y Acad Sci. 2014. PMID: 24673341 Free PMC article. Review. - Diabetic retinopathy: Role of inflammation and potential therapies for anti-inflammation.
Liou GI. Liou GI. World J Diabetes. 2010 Mar 15;1(1):12-8. doi: 10.4239/wjd.v1.i1.12. World J Diabetes. 2010. PMID: 21537423 Free PMC article.
References
- Antonetti DA, Barber AJ, Khin S, Lieth E, Tarbell JM, Gardner TW: Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content. Diabetes 1998, 47:1953-1959 - PubMed
- Adamis AP, Miller JW, Bernal MT, D’Amico DJ, Folkman J, Yeo TK, Yeo KT: Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol 1994, 118:445-450 - PubMed
- Qaum T, Xu Q, Houssen A, Clemens MW, Qin W, Miyamoto K, Hassessian H, Wiegand SJ, Rudge J, Yancopoulos GD, Adamis AP: VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest Ophthalmol Vis Sci 2001, 42:2408-2413 - PubMed
- Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE: Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 1994, 331:1480-1487 - PubMed
- Takeda M, Mori F, Yoshida A, Takamiya A, Nakagomi S, Sato E, Kiyama H: Constitutive nitric oxide synthase is associated with retinal vascular permeability in early diabetic rats. Diabetologia 2001, 44:1043-1050 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources