Nonpathogenic Escherichia coli can contribute to the production of Shiga toxin - PubMed (original) (raw)
Nonpathogenic Escherichia coli can contribute to the production of Shiga toxin
Shantini D Gamage et al. Infect Immun. 2003 Jun.
Abstract
The food-borne pathogen, Escherichia coli O157:H7, has been associated with gastrointestinal disease and the life-threatening sequela hemolytic uremic syndrome. The genes for the virulence factor, Shiga toxin 2 (Stx2), in E. coli O157:H7 are encoded on a temperate bacteriophage under the regulation of the late gene promoter. Induction of the phage lytic cycle is required for toxin synthesis and release. We investigated the hypothesis that nonpathogenic E. coli could amplify Stx2 production if infected with the toxin-encoding phage. Toxin-encoding phage were incubated with E. coli that were either susceptible or resistant to the phage. The addition of phage to phage-susceptible bacteria resulted in up to 40-fold more toxin than a pure culture of lysogens, whereas the addition of phage to phage-resistant bacteria resulted in significantly reduced levels of toxin. Intestinal E. coli isolates incubated with Shiga toxin-encoding phage produced variable amounts of toxin. Of 37 isolates, 3 produced significantly more toxin than was present in the inoculum, and 1 fecal isolate appeared to inactivate the toxin. Toxin production in the intestine was assessed in a murine model. Fecal toxin recovery was significantly reduced when phage-resistant E. coli was present. These results suggest that the susceptibility of the intestinal flora to the Shiga toxin phage could exert either a protective or an antagonistic influence on the severity of disease by pathogens with phage-encoded Shiga toxin. Toxin production by intestinal flora may represent a novel strategy of pathogenesis.
Figures
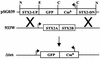
FIG. 1.
Construction of Δtox. The Stx2 genes in phage 933W were replaced with GFP and Cmr genes. Expression of GFP is under the control of the phage late gene promoter, and Cmr expression is under the control of its own promoter. N, _Not_I; E, _Eco_RI; C, _Cla_I.
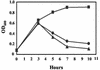
FIG. 2.
Phage-induced lysis after treatment with ciprofloxacin. Cultures of C600 and C600 lysogenized with either wild-type 933W or mutant Δtox were treated with 30 ng of ciprofloxacin/ml for 10 h. The OD600 was measured as an indication of bacterial lysis due to phage production. Symbols: ▪, C600; •, C600::933W; ▴, C600::Δtox.
FIG. 3.
Western analysis of GFP production by C600::Δtox. GFP in culture supernatants was examined. Lane 1, GFP protein from pGFPuv; lane 2, C600::Δtox induced with ciprofloxacin; lane 3, C600::933W induced with ciprofloxacin; lane 4, uninduced C600::Δtox. Lanes 5 to 11: C600::Δtox incubated alone or with 933W phage as indicated: no phage added (lane 5), 105 phage added (lane 6), 104 phage added (lane 7), 103 phage added (lane 8), 102 phage added (lane 9), 101 phage added (lane 10), or 100 phage added (lane 11).
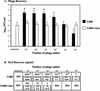
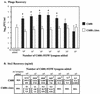
FIG. 4.
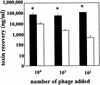
Phage (A) and toxin (B) recovery after infection with 933W phage. The results are the average of four trials. (A) Phage recovery (log10 PFU/milliliter + the SEM). An asterisk denotes statistically different values (P < 0.05 [Student t test]) of log10 phage counts from C600 compared to log10 counts from C600::Δtox for each challenge dose. (B) Toxin recovery. The top number is the geometric mean Stx2; the number in brackets represents the 95% confidence level of the mean. An asterisk indicates statistical significance (P < 0.05 [Student t test of log toxin values]) of Stx2 from C600 compared to C600::Δtox for each challenge dose. BDL, below detection limit of 0.15 ng/ml.
FIG. 5.
Phage (A) and toxin (B) recovery after coincubation with C600::933W. The results are the average of four trials. (A) Phage recovery (log10 PFU/milliliter + the SEM). An asterisk denotes statistically different values (P < 0.05 [Student t test]) from C600 compared to C600::Δtox for each challenge dose. (B) Toxin recovery. The top number is the geometric mean Stx2; the number in brackets represents the 95% confidence levels of the mean. An asterisk indicates statistical significance (P < 0.05 [Student t test of log toxin values]) of Stx2 from C600 compared to C600::Δtox for each challenge dose. BDL, below detection limit of 0.15 ng/ml.
FIG. 6.
Toxin recovery after incubation with phage from E. coli O157:H7. A total of 109 CFU of either C600 (▪) or C600::Δtox (□)/ml was incubated overnight with various dilutions of Shiga toxin-encoding phage from E. coli O157:H7 strain PT-32. The toxin concentration in the supernatant (nanograms/milliliter + the SEM) was determined by Vero cell assay. An asterisk indicates statistically different values (P < 0.05 [Student t test]) from C600 compared to C600::Δtox for each challenge dose. The results are the average of three trials.
FIG. 7.
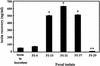
Toxin recovery after incubation of human intestinal E. coli with E. coli O157:H7. A total of 109 CFU of fecal E. coli/ml was incubated overnight with 100 phage from E. coli O157:H7 PT-32. The values represent toxin recovery (nanograms/milliliter) + the SEM. An asterisk denotes significantly (P < 0.01) greater toxin production compared to toxin present in the initial phage inoculum. A double asterisk denotes significantly (P < 0.01) less toxin production compared to toxin in the initial phage inoculum. The results are the average of three trials.
FIG. 8.
Model of the effect of normal intestinal E. coli on the production of Stx2 by E. coli O157:H7. During an E. coli O157:H7 infection, lysis of the pathogen occurs in the intestine, releasing a basal level of Stx2-encoding phage and toxin. If the normal E. coli in the intestine is susceptible to infection by the Shiga toxin-encoding phage, repeated cycles of infection and lysis will occur, resulting in an amplification of toxin production. If the normal intestinal E. coli are resistant to infection by the Shiga toxin-encoding phage, toxin production will be limited to the E. coli O157:H7, and only basal levels of toxin will be produced.
Similar articles
- Cinnamon Oil Inhibits Shiga Toxin Type 2 Phage Induction and Shiga Toxin Type 2 Production in Escherichia coli O157:H7.
Sheng L, Rasco B, Zhu MJ. Sheng L, et al. Appl Environ Microbiol. 2016 Oct 27;82(22):6531-6540. doi: 10.1128/AEM.01702-16. Print 2016 Nov 15. Appl Environ Microbiol. 2016. PMID: 27590808 Free PMC article. - Diversity and host range of Shiga toxin-encoding phage.
Gamage SD, Patton AK, Hanson JF, Weiss AA. Gamage SD, et al. Infect Immun. 2004 Dec;72(12):7131-9. doi: 10.1128/IAI.72.12.7131-7139.2004. Infect Immun. 2004. PMID: 15557637 Free PMC article. - Vitamin K Analogs Influence the Growth and Virulence Potential of Enterohemorrhagic Escherichia coli.
Kijewski A, Witsø IL, Iversen H, Rønning HT, L'Abée-Lund T, Wasteson Y, Lindbäck T, Aspholm M. Kijewski A, et al. Appl Environ Microbiol. 2020 Nov 24;86(24):e00583-20. doi: 10.1128/AEM.00583-20. Print 2020 Nov 24. Appl Environ Microbiol. 2020. PMID: 32769190 Free PMC article. - Commensal bacteria influence Escherichia coli O157:H7 persistence and Shiga toxin production in the mouse intestine.
Gamage SD, Patton AK, Strasser JE, Chalk CL, Weiss AA. Gamage SD, et al. Infect Immun. 2006 Mar;74(3):1977-83. doi: 10.1128/IAI.74.3.1977-1983.2006. Infect Immun. 2006. PMID: 16495578 Free PMC article. - A Toxic Environment: a Growing Understanding of How Microbial Communities Affect Escherichia coli O157:H7 Shiga Toxin Expression.
Nawrocki EM, Mosso HM, Dudley EG. Nawrocki EM, et al. Appl Environ Microbiol. 2020 Nov 24;86(24):e00509-20. doi: 10.1128/AEM.00509-20. Print 2020 Nov 24. Appl Environ Microbiol. 2020. PMID: 32358004 Free PMC article. Review.
Cited by
- Evolutionary ecology of microbial wars: within-host competition and (incidental) virulence.
Brown SP, Fredrik Inglis R, Taddei F. Brown SP, et al. Evol Appl. 2009 Feb;2(1):32-9. doi: 10.1111/j.1752-4571.2008.00059.x. Epub 2009 Jan 7. Evol Appl. 2009. PMID: 25567845 Free PMC article. - Lysogenic conversion and phage resistance development in phage exposed Escherichia coli biofilms.
Moons P, Faster D, Aertsen A. Moons P, et al. Viruses. 2013 Jan 11;5(1):150-61. doi: 10.3390/v5010150. Viruses. 2013. PMID: 23344561 Free PMC article. - Induction of Shiga toxin-converting prophage in Escherichia coli by high hydrostatic pressure.
Aertsen A, Faster D, Michiels CW. Aertsen A, et al. Appl Environ Microbiol. 2005 Mar;71(3):1155-62. doi: 10.1128/AEM.71.3.1155-1162.2005. Appl Environ Microbiol. 2005. PMID: 15746313 Free PMC article. - Cationic Antimicrobial Peptides Inactivate Shiga Toxin-Encoding Bacteriophages.
Del Cogliano ME, Hollmann A, Martinez M, Semorile L, Ghiringhelli PD, Maffía PC, Bentancor LV. Del Cogliano ME, et al. Front Chem. 2017 Dec 19;5:122. doi: 10.3389/fchem.2017.00122. eCollection 2017. Front Chem. 2017. PMID: 29312928 Free PMC article. - The Probiotic Escherichia coli Strain Nissle 1917 Combats Lambdoid Bacteriophages stx and λ.
Bury S, Soundararajan M, Bharti R, von Bünau R, Förstner KU, Oelschlaeger TA. Bury S, et al. Front Microbiol. 2018 May 29;9:929. doi: 10.3389/fmicb.2018.00929. eCollection 2018. Front Microbiol. 2018. PMID: 29896160 Free PMC article.
References
- Besser, R. E., P. M. Griffin, and L. Slutsker. 1999. Escherichia coli O157:H7 gastroenteritis and the hemolytic uremic syndrome: an emerging infectious disease. Annu. Rev. Med. 50:355-367. - PubMed
- Dykstra, S. A., R. A. Moxley, B. H. Janke, E. A. Nelson, and D. H. Francis. 1993. Clinical signs and lesions in gnotobiotic pigs inoculated with Shiga-like toxin I from Escherichia coli. Vet. Pathol. 30:410-417. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI023695/AI/NIAID NIH HHS/United States
- R21 AI054762/AI/NIAID NIH HHS/United States
- R01 AI23695/AI/NIAID NIH HHS/United States
- R21 AI54762/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources