Frequency modulation of synchronized Ca2+ spikes in cultured hippocampal networks through G-protein-coupled receptors - PubMed (original) (raw)
Frequency modulation of synchronized Ca2+ spikes in cultured hippocampal networks through G-protein-coupled receptors
Zhijun Liu et al. J Neurosci. 2003.
Abstract
Synchronized spontaneous Ca2+ spikes in networked neurons represent periodic burst firing of action potentials, which are believed to play a major role in the development and plasticity of neuronal circuitry. How these network activities are shaped and modulated by extrinsic factors during development, however, remains to be studied. Here we report that synchronized Ca2+ spikes among cultured hippocampal neurons can be modulated by two small factors that act on G-protein-coupled receptors (GPCRs): the neuropeptide PACAP (pituitary adenylate cyclase-activating polypeptide) and the chemokine SDF-1 (stromal cell-derived factor-1). PACAP effectively increases the frequency of the synchronized Ca2+ spikes when applied acutely; the PACAP potentiation of Ca2+ spikes requires the activation of the PACAP-specific PAC1 GPCRs and is mediated by the activation of cAMP signaling pathway. SDF-1, on the other hand, significantly reduces the frequency of these Ca2+ spikes through the activation of its specific GPCR CXCR4; the inhibitory action of SDF-1 is mediated by the inhibition of cAMP pathway through the Gi component of GPCRs. Taken together, these results demonstrate that synchronized neuronal network activity can be effectively modulated by physiologically and developmentally relevant small factors that act on GPCRs to target the cAMP pathway. Such modulation of neuronal activity through GPCRs may represent a significant mechanism that underlies the neuronal plasticity during neural development and functioning.
Figures
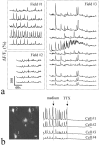
Figure 1.
Synchronized spontaneous Ca2+ spikes in cultured hippocampal neurons. a, Representative recordings of synchronized spontaneous Ca2+ spikes from hippocampal neurons 2 weeks in culture. Traces indicate relative changes of fluo-3 intensity (Δ_F_/F_0) over time in neurons from three microscopic fields randomly selected from the same culture dish. Each trace represents Δ_F/_F_0 of an individual neuron acquired every 2 sec (see Materials and Methods). b, Blockade of spontaneous synchronized Ca2+ spikes by TTX. The fluorescent image on the left shows four hippocampal neurons loaded with fluo-3. Scale bar, 5μm. Traces on the right depict Ca2+ spikes in these cells under control conditions, after application of control medium, and subsequent application of TTX (1 μ
m
final).
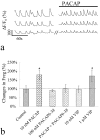
Figure 2.
Increase in the frequency of synchronized Ca2+ spike firing induced by PACAP. a, Traces show synchronized Ca2+ spikes in three neurons randomly selected from a group of synchronically firing cells before and after bath addition of PACAP (10 n
m
). The time gap (//) is 5 min. b, Changes of the synchronized Ca2+ spike frequency 5–6 min after bath application of a different molecule or molecules. The change of the Ca2+ spike frequency was quantified by normalizing the spike number from a 2 min period (5–6 min) after bath application to the spike number in the control period and presented as the percentage. Data are presented as the mean ± SD from many cells (PACAP: n = 24; PACAP6–38: n = 21; PACAP + PACAP6–38: n = 22; 10 n
m
VIP: n = 22; 1 μ
m
VIP: n = 23, PACAP6–38). Asterisks indicate significance against the control (p < 0.005, t test).
Figure 3.
Increased frequency of firing of hippocampal neurons by PACAP, as recorded by the whole-cell patch-clamp technique. a, Effects of PACAP on EPSCs in a hippocampal neuron from a 1 week culture. The trace represents the spontaneous EPSCs of a hippocampal neuron from a 1 week culture before and after bath application of 10 n
m
PACAP. The shape of each EPSC is depicted by the expanded traces. To determine the changes of the EPSC number over time, we counted the numbers of EPSCs every 1 min before and after the addition of PACAP (b). c, The increase in the number of EPSCs after PACAP addition was quantified by normalizing EPSC number from a 2 min period to that of the control period. Data represent mean ± SD.
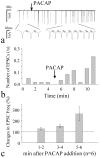
Figure 4.
cAMP dependence of PACAP enhancement of Ca2+ spike frequency. a, Modulation of the frequency of spontaneous Ca2+ spikes by manipulation of cAMP signaling pathway. Traces show representative recordings of synchronized Ca2+ spikes in three hippocampal neurons randomly selected from the imaging field under baseline and after bath application of KT5720 (1 μ
m
). b, Effects of different PAK antagonists and agonist on the frequency of the synchronized Ca2+ spikes. As described previously, the modulation was quantified by normalizing the number of spikes in 5–6 min after bath application to that of the control period. Data represent mean ± SD from populations of cells (1μ
m
KT5720: n = 9; 200 μ
m
Rp-cAMP: n = 21; 10 n
m
PACAP + Rp-cAMP: n = 18; 15 μ
m
forskolin: n = 20). *p < 0.005 to the control group treated by control medium (t test). c, Traces indicate the Ca2+ spikes in three hippocampal neurons in the presence of 1 μ
m
KT 5720 before and after PACAP application (10 n
m
). d, Bar graph summarizes the blockade of PACAP effects on Ca2+ spike frequency by KT5720 or Rp-cAMP.
Figure 5.
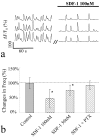
Inhibition of synchronized Ca2+ spikes by SDF-1. a, Representative recordings of synchronized Ca2+spikes in three hippocampal neurons randomly selected in the imaging field before and after addition of SDF-1(100n
m
). The time gap (//) indicates ∼5 min. _b,_The decrease of the Ca2+ spike frequency by SDF-1 is quantified by normalizing the number of spikes from 5–6 min after SDF-1 application to the control period. Data represent mean ± SD from populations of cells (100 n
m
SDF-1:_n_=35; 50 n
m
SDF-1,_n_=15). For PTX experiments, hippocampal cultures were pretreated with 200 ng/ml PTX for 16 hr before Ca2+ imaging (n = 11). *p < 0.005 to the control values treated by control medium (t test).
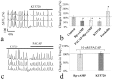
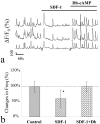
Figure 6.
cAMP dependence of SDF-1 inhibition of synchronized spontaneous Ca2+ spikes. a, Representative recordings of synchronized Ca2+ spike in three hippocampal neurons under baseline control conditions, in the presence of SDF-1 (100 n
m
), and after subsequent addition of 500 μ
m
dibutyryl cAMP. b, The bar graph depicts the normalized frequency changes of control (saline application), SDF-1 addition, and after subsequent addition of db-cAMP. Data represent mean ± SD from 13 neurons of three different dishes. *p < 0.005 to the control values treated by control medium (t test).
Similar articles
- Modulation of AMPA receptor-mediated ion current by pituitary adenylate cyclase-activating polypeptide (PACAP) in CA1 pyramidal neurons from rat hippocampus.
Costa L, Santangelo F, Li Volsi G, Ciranna L. Costa L, et al. Hippocampus. 2009 Jan;19(1):99-109. doi: 10.1002/hipo.20488. Hippocampus. 2009. PMID: 18727050 - Pituitary adenylate cyclase-activating polypeptide potentiation of Ca2+ entry via protein kinase C and A pathways in melanotrophs of the pituitary pars intermedia of rats.
Tanaka K, Shibuya I, Harayama N, Nomura M, Kabashima N, Ueta Y, Yamashita H. Tanaka K, et al. Endocrinology. 1997 Oct;138(10):4086-95. doi: 10.1210/endo.138.10.5442. Endocrinology. 1997. PMID: 9322916 - Convergent phosphomodulation of the major neuronal dendritic potassium channel Kv4.2 by pituitary adenylate cyclase-activating polypeptide.
Gupte RP, Kadunganattil S, Shepherd AJ, Merrill R, Planer W, Bruchas MR, Strack S, Mohapatra DP. Gupte RP, et al. Neuropharmacology. 2016 Feb;101:291-308. doi: 10.1016/j.neuropharm.2015.10.006. Epub 2015 Oct 9. Neuropharmacology. 2016. PMID: 26456351 Free PMC article. - Pituitary adenylate cyclase-activating polypeptide (PACAP) and its receptors in the brain.
Shioda S. Shioda S. Kaibogaku Zasshi. 2000 Dec;75(6):487-507. Kaibogaku Zasshi. 2000. PMID: 11197592 Review. - Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems.
Arimura A. Arimura A. Jpn J Physiol. 1998 Oct;48(5):301-31. doi: 10.2170/jjphysiol.48.301. Jpn J Physiol. 1998. PMID: 9852340 Review.
Cited by
- Pituitary Adenylate Cyclase-Activating Polypeptide Modulates Hippocampal Synaptic Transmission and Plasticity: New Therapeutic Suggestions for Fragile X Syndrome.
Ciranna L, Costa L. Ciranna L, et al. Front Cell Neurosci. 2019 Nov 27;13:524. doi: 10.3389/fncel.2019.00524. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31827422 Free PMC article. Review. - Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a.
Yermolaieva O, Leonard AS, Schnizler MK, Abboud FM, Welsh MJ. Yermolaieva O, et al. Proc Natl Acad Sci U S A. 2004 Apr 27;101(17):6752-7. doi: 10.1073/pnas.0308636100. Epub 2004 Apr 13. Proc Natl Acad Sci U S A. 2004. PMID: 15082829 Free PMC article. - Chronic CXCL10 alters neuronal properties in rat hippocampal culture.
Cho J, Nelson TE, Bajova H, Gruol DL. Cho J, et al. J Neuroimmunol. 2009 Feb 15;207(1-2):92-100. doi: 10.1016/j.jneuroim.2008.12.007. Epub 2009 Jan 22. J Neuroimmunol. 2009. PMID: 19167097 Free PMC article. - Self-Organized Synchronous Calcium Transients in a Cultured Human Neural Network Derived from Cerebral Organoids.
Sakaguchi H, Ozaki Y, Ashida T, Matsubara T, Oishi N, Kihara S, Takahashi J. Sakaguchi H, et al. Stem Cell Reports. 2019 Sep 10;13(3):458-473. doi: 10.1016/j.stemcr.2019.05.029. Epub 2019 Jun 27. Stem Cell Reports. 2019. PMID: 31257131 Free PMC article. - Growth factors in ischemic stroke.
Lanfranconi S, Locatelli F, Corti S, Candelise L, Comi GP, Baron PL, Strazzer S, Bresolin N, Bersano A. Lanfranconi S, et al. J Cell Mol Med. 2011 Aug;15(8):1645-87. doi: 10.1111/j.1582-4934.2009.00987.x. Epub 2009 Dec 8. J Cell Mol Med. 2011. PMID: 20015202 Free PMC article. Review.
References
- Abel T, Kandel E ( 1998) Positive and negative regulatory mechanisms that mediate long-term memory storage. Brain Res Brain Res Rev 26: 360–378. - PubMed
- Anholt RR ( 1994) Signal integration in the nervous system: adenylate cyclases as molecular coincidence detectors. Trends Neurosci 17: 37–41. - PubMed
- Asensio VC, Campbell IL ( 1999) Chemokines in the CNS: plurifunctional mediators in diverse states. Trends Neurosci 22: 504–512. - PubMed
- Bacci A, Verderio C, Pravettoni E, Matteoli M ( 1999) Synaptic and intrinsic mechanisms shape synchronous oscillations in hippocampal neurons in culture. Eur J Neurosci 11: 389–397. - PubMed
- Banker GA, Cowan WM ( 1977) Rat hippocampal neurons in dispersed cell culture. Brain Res 126: 397–425. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous