Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice - PubMed (original) (raw)
. 2003 Jan-Feb;9(1-2):10-7.
Affiliations
- PMID: 12765335
- PMCID: PMC1430379
Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice
Giuseppina Caligiuri et al. Mol Med. 2003 Jan-Feb.
Abstract
Interleukin (IL)-10 is an anti-inflammatory cytokine that may play a protective role in atherosclerosis. The aim of this study was to assess the effect of IL-10 deficiency in the apolipoprotein E knockout mouse. Apolipoprotein E deficient (E-/-) and IL-10 deficient (-/-) mice were crossed to generate E-/- x IL-10-/- double knockout mice. By 16 wk, cholesterol and triglycerides were similar in double and single knockouts but the lack of IL-10 led to increased low-density lipoprotein cholesterol whereas very-low-density lipoprotein was reduced. In parallel, T-helper 1 responses and lesion size were dramatically increased in double knockout compared with E-/- controls. At 48 wk, matrix metalloproteinases and tissue factor activities were increased in lesions of double-knockout mice. Furthermore, markers of systemic coagulation were increased, and vascular thrombosis in response to i.v. thrombin occurred more frequently in E-/- x IL-10-/- than in E-/- mice. Our findings suggest that IL-10 deficiency plays a deleterious role in atherosclerosis. The early phase of lesion development was increased, and the proteolytic and procoagulant activity was elevated in advanced lesions. These data show that IL-10 may reduce atherogenesis and improve the stability of plaques.
Figures
Figure 1
Computer-assisted morphometry was applied to aortic cryosections, stained with oil red O, harvested at the level of the aortic cusps. Data show lesion size (surface area of lesions/surface area of vessel, %) and were averaged from 4 sections per mouse (400, 500, 600, and 700 μm from the appearance of the 1st cusp). —, mean; *P < 0.05, female compared with male mice; †P < 0.05, E−/− × IL-10−/− compared with E−/− × IL-10+/+ mice.
Figure 2
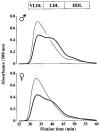
Plasma lipoprotein patterns of 16-wk-old male (A) and female (B) mice following separation by FPLC. Lines represent means and gray sections represent SEM. A: Thin line, 7 male E−/− × IL-10+/+ mice; thick line, 9 male E−/− × IL-10−/− mice. B: Thin line, 4 female E−/− × IL-10+/+ mice; thick line, 4 female E−/− × IL-10−/− mice. In both panels, light gray areas show the results of 2 different human samples, after 4 repetitive injections (before, after, and between mouse samples) to monitor assay drift over time and to show elution profile for human.
Figure 3
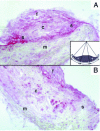
MMP 9 localization in plaques from E−/− × IL-10−/− (A) and E−/− × IL-10+/+ (B) mice. Original magnification ×200; c, core; f, fibrous cap; m, media; s, shoulder region. Note MMP 9 accumulates in the shoulder regions in E−/− × IL-10−/− and not in E−/− × IL-10+/+ mice. Quantification was performed by computer-assisted morphometry. Plaques were divided into 3 portions as indicated in the insert (s, shoulder). The optical density within shoulder regions and the optical density within the total surface area of the plaque were computed.
Figure 4
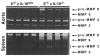
Gelatinolytic activities (zymography) of aortas and spleen mononuclear cells from 4 E−/− × IL-10+/+ and 4 E−/− × IL-10−/− mice. Samples were prepared as described in Materials and Methods. Gels were submitted to scanning densitometry analysis (see Table 2).
Figure 5
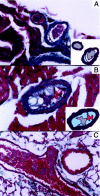
Platelet arterial thrombi in thrombin-injected E−/− × IL-10−/−mice. Masson’s trichrome, magnification ×200 (A,B) and ×100 (C). A: Platelet thrombi occupy the lumen of 2 small epicardial branches of the left anterior descending coronary artery. An atherosclerotic plaque is evident in 1 of 2 thrombosed arteries. Insert: A model depicts the thrombus (gray) and the plaque (light blue). B: A thrombus occluding the lumen of an atherosclerotic intramyocardial coronary branch. Insert: A model depicts the platelet-rich part of the thrombus (gray), the erythrocytes (red), and the plaque (light blue). C: An occlusive red clot in a pulmonary artery of a mouse that died within 5 min of intravenous thrombin injection.
Similar articles
- Reduced atherosclerosis in interleukin-18 deficient apolipoprotein E-knockout mice.
Elhage R, Jawien J, Rudling M, Ljunggren HG, Takeda K, Akira S, Bayard F, Hansson GK. Elhage R, et al. Cardiovasc Res. 2003 Jul 1;59(1):234-40. doi: 10.1016/s0008-6363(03)00343-2. Cardiovasc Res. 2003. PMID: 12829194 - Combined serum paraoxonase knockout/apolipoprotein E knockout mice exhibit increased lipoprotein oxidation and atherosclerosis.
Shih DM, Xia YR, Wang XP, Miller E, Castellani LW, Subbanagounder G, Cheroutre H, Faull KF, Berliner JA, Witztum JL, Lusis AJ. Shih DM, et al. J Biol Chem. 2000 Jun 9;275(23):17527-35. doi: 10.1074/jbc.M910376199. J Biol Chem. 2000. PMID: 10748217 - Hypercholesterolemia is associated with a T helper (Th) 1/Th2 switch of the autoimmune response in atherosclerotic apo E-knockout mice.
Zhou X, Paulsson G, Stemme S, Hansson GK. Zhou X, et al. J Clin Invest. 1998 Apr 15;101(8):1717-25. doi: 10.1172/JCI1216. J Clin Invest. 1998. PMID: 9541503 Free PMC article. - Mouse models of experimental atherosclerosis.
Jawień J, Nastałek P, Korbut R. Jawień J, et al. J Physiol Pharmacol. 2004 Sep;55(3):503-17. J Physiol Pharmacol. 2004. PMID: 15381823 Review. - Use of transgenic mice to study the role of apolipoprotein E in lipid metabolism and atherosclerosis.
Willems Van Dijk K, Hofker MH, Havekes LM. Willems Van Dijk K, et al. Int J Tissue React. 2000;22(2-3):49-58. Int J Tissue React. 2000. PMID: 10937354 Review.
Cited by
- MiR-6721-5p as a natural regulator of Meta-VCL is upregulated in the serum of patients with coronary artery disease.
Gholipour A, Zahedmehr A, Arabian M, Shakerian F, Maleki M, Oveisee M, Malakootian M. Gholipour A, et al. Noncoding RNA Res. 2024 Aug 27;10:25-34. doi: 10.1016/j.ncrna.2024.08.006. eCollection 2025 Feb. Noncoding RNA Res. 2024. PMID: 39296643 Free PMC article. - Major Adverse Cardiovascular Events: The Importance of Serum Levels and Haplotypes of the Anti-Inflammatory Cytokine Interleukin 10.
Schulz S, Reuter L, Navarrete Santos A, Bitter K, Rehm S, Schlitt A, Reichert S. Schulz S, et al. Biomolecules. 2024 Aug 9;14(8):979. doi: 10.3390/biom14080979. Biomolecules. 2024. PMID: 39199367 Free PMC article. - Aberrant mitochondrial DNA synthesis in macrophages exacerbates inflammation and atherosclerosis.
Natarajan N, Florentin J, Johny E, Xiao H, O'Neil SP, Lei L, Shen J, Ohayon L, Johnson AR, Rao K, Li X, Zhao Y, Zhang Y, Tavakoli S, Shiva S, Das J, Dutta P. Natarajan N, et al. Nat Commun. 2024 Aug 26;15(1):7337. doi: 10.1038/s41467-024-51780-1. Nat Commun. 2024. PMID: 39187565 Free PMC article. - Targeting immune cell recruitment in atherosclerosis.
Döring Y, van der Vorst EPC, Weber C. Döring Y, et al. Nat Rev Cardiol. 2024 Nov;21(11):824-840. doi: 10.1038/s41569-024-01023-z. Epub 2024 Apr 25. Nat Rev Cardiol. 2024. PMID: 38664575 Review. - The diverse roles of macrophages in metabolic inflammation and its resolution.
Guha Ray A, Odum OP, Wiseman D, Weinstock A. Guha Ray A, et al. Front Cell Dev Biol. 2023 Mar 13;11:1147434. doi: 10.3389/fcell.2023.1147434. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36994095 Free PMC article. Review.
References
- Hansson GK. Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:1876–90. - PubMed
- Mallat Z, et al. Protective role of interleukin-10 in atherosclerosis. Circ Res. 1999;85:e17–24. - PubMed
- Pinderski LJ, et al. Overexpression of interleukin-10 by activated T lymphocytes inhibits atherosclerosis in LDL receptor-deficient mice by altering lymphocyte and macrophage phenotypes. Circ Res. 2002;90:1064–71. - PubMed
- Pinderski Oslund LJ, et al. Interleukin-10 blocks atherosclerotic events in vitro and in vivo. Arterioscler Thromb Vasc Biol. 1999;19:2847–53. - PubMed
- Laurat E, et al. In vivo downregulation of T helper cell 1 immune responses reduces atherogenesis in apolipoprotein E-knockout mice. Circulation. 2001;104:197–202. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases