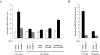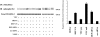Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148 - PubMed (original) (raw)
Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148
Maria Grazia Lampugnani et al. J Cell Biol. 2003.
Abstract
Confluent endothelial cells respond poorly to the proliferative signals of VEGF. Comparing isogenic endothelial cells differing for vascular endothelial cadherin (VE-cadherin) expression only, we found that the presence of this protein attenuates VEGF-induced VEGF receptor (VEGFR) 2 phosphorylation in tyrosine, p44/p42 MAP kinase phosphorylation, and cell proliferation. VE-cadherin truncated in beta-catenin but not p120 binding domain is unable to associate with VEGFR-2 and to induce its inactivation. beta-Catenin-null endothelial cells are not contact inhibited by VE-cadherin and are still responsive to VEGF, indicating that this protein is required to restrain growth factor signaling. A dominant-negative mutant of high cell density-enhanced PTP 1 (DEP-1)//CD148 as well as reduction of its expression by RNA interference partially restore VEGFR-2 phosphorylation and MAP kinase activation. Overall the data indicate that VE-cadherin-beta-catenin complex participates in contact inhibition of VEGF signaling. Upon stimulation with VEGF, VEGFR-2 associates with the complex and concentrates at cell-cell contacts, where it may be inactivated by junctional phosphatases such as DEP-1. In sparse cells or in VE-cadherin-null cells, this phenomenon cannot occur and the receptor is fully activated by the growth factor.
Figures
Figure 1.
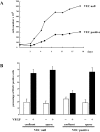
Confluence and VE-cadherin expression inhibit endothelial proliferation induced by VEGF. (A) Growth curve of endothelial cells expressing (VEC positive) or not expressing (VEC null) VE-cadherin. Cells (seeding 30,000/cm2) were cultured in complete culture medium. At the indicated time point, cells were detached and counted. The standard deviation in three independent experiments was between 5 and 10% of the mean values. (B) Confluent (100,000/cm2) and sparse (20,000/cm2) VEC-positive and VEC-null cells were stimulated with VEGF (80 ng/ml) for 24 h. BrdU (30 μM) was added during the last 4 h. A total of 300 nuclei in random fields for each treatment were scored. The mean values of three independent experiments ± SD are shown.
Figure 2.
VE-cadherin expression and clustering inhibit VEGFR-2 tyrosine phosphorylation. (A) Confluent VEC-null and -positive endothelial cells were stimulated with VEGF (80 ng/ml) for the indicated time intervals. Cell extracts were immunoprecipitated with antibodies to VEGFR-2 (IP αVEGFR-2) and immunoblotted (IB) with antibodies to phosphotyrosine (αphosphoTyr) and VEGFR-2 (αVEGFR-2). A similar experimental procedure was used for B–D. In the representative experiment shown, tyrosine-phosphorylated VEGFR-2 normalized over total VEGFR-2 was 0.5-, five-, and twofold more in VEC-null than in VEC-positive cells at time 0, 5, and 30 min, respectively. In 15 independent experiments, the range of increase at 5 min was from two- to sevenfold. (B) Tyrosine phosphorylation of VEGFR-2 in response to VEGF (80 ng/ml for 5 min) in sparse VEC-null and -positive endothelial cells was comparable. (C) In HUVEC, phosphorylation of VEGFR-2 in response to VEGF (80 ng/ml for 5 min) was lower in confluent than in sparse cultures (range three- to fivefold lower in four experiments). (D) Addition of antibodies to VE-cadherin (anti-VEC, 100 μg/ml) for 1 h increased receptor phosphorylation by VEGF (80 ng/ml, for 5 min). In response to VEGF, the phosphotyrosine content in VEGFR-2 was higher (from three- to fourfold in three experiments) in cells pretreated with VE-cadherin antibody. The antibody to VEGFR-2 recognized two bands at a molecular mass of ∼200 kD. Only the higher molecular mass band, representing the mature form of the receptor, was phosphorylated in tyrosine, as also described by Takahashi and Shibuya (1997). In the following figures, we therefore show only the heavier band of the doublet, which represents the phosphorylable pool of VEGFR-2.
Figure 3.
VE-cadherin expression and clustering reduce the extent of p44/42 MAP kinase phosphorylation in response to VEGF. (A) Confluent and sparse VEC-null and VEC-positive endothelial cells were stimulated with VEGF (80 ng/ml for 10 min), and phospho p44/42 MAP kinase and total MAP kinase were assayed by Western blot with specific antibodies. The columns represent the ratio between phosphorylated and total values as fold increase over the ratio calculated in sparse unstimulated VEC null. VEGF-stimulated phosphorylation of MAP kinases was reduced at confluence only in VEC-positive cells. The mean ± SD of three independent experiments is reported. In a total of 12 experiments, the increase of p44/42 MAP kinase phosphorylation in VEC-null cells ranged from three- to sixfold over VEC-positive cells. (B) In confluent HUVEC, phosphorylation of p44/42 MAP kinase in response to VEGF (80 ng/ml for 10 min) was reduced about threefold in comparison with sparse cells. Column values are as in A.
Figure 4.
Inhibition of p44/42 MAP kinase phosphorylation correlates with reduction of BrdU incorporation in VEC-null endothelial cells. (A) Confluent VEC-null or -positive cells were treated with PD98059 (100 μM) for 20 min before challenge with VEGF (80 ng/ml for 10 min). VEC-positive cells were transfected with a constitutive active or wild-type MAP kinase kinase (MAPKK) construct or the empty vector. Nuclear incorporation of BrdU was evaluated after 24 h as in the legend to Fig. 1. PD98059 strongly inhibited proliferation of VEC-null cells in response to VEGF. Proliferation of VEC-positive cells was very low and was inhibited by the drug. Constitutively active MAPK kinase partially restored BrdU incorporation in response to VEGF in VEC-positive cells. (B) The concentration of PD98059 used in A was able to strongly inhibit phosphorylation of p44/42 MAP kinase after a 10-min stimulation with VEGF (80 ng/ml). Each column represents the fold increase of the ratio between phosphorylated and total values in VEGF-stimulated over the respective unstimulated condition. Data are means ± SD of three independent experiments. The vehicle is DMSO added at the same final concentration (0.1%) as in PD98059-treated cells.
Figure 5.
VE-cadherin expression down-regulates tyrosine phosphorylation of VEGFR-2 and phosphorylation of p44/42 MAP kinase over a range of VEGF concentrations. Confluent VEC-null and -positive endothelial cells were challenged for 5 min with different concentrations of VEGF (0.8, 8, and 80 ng/ml). Phosphorylated and total VEGFR-2 (A) and p44/42 MAP kinase (B) were assayed as described in the legend to Figs. 2 and 3. The fold increases of receptor and p44/42 MAP kinase phosphorylation in respect to unstimulated cells are reported on the right side of each panel. The SD was 5–15% of the means (three independent experiments).
Figure 6.
VEGFR-2 association and dephosphorylation requires the β-catenin binding domain of VE-cadherin. VEC-null cells were transfected with VE-cadherin wild type or truncated mutants lacking β-catenin (Δ-βcat) or p120 (Δ-p120) binding domains. Intra, intracellular region; extra, extracellular region (C). (A) After stimulation with VEGF (80 ng/ml) for 5 and 30 min, cell extracts were immunoprecipitated (IP) with antibodies to VEGFR-2 (αVEGFR-2) and immunoblotted (IB) with antibodies to phosphotyrosine (αphosphoTyr), VEGFR-2 (αVEGFR-2), and VE-cadherin (αVEC). Wild-type (molecular mass, ∼120 kD) and Δ-p120 VE-cadherin (molecular mass, ∼100 kD) were coimmunoprecipitated with VEGFR-2 (A, lower panel). Receptor phosphorylation was significantly reduced in VEC-positive and Δ-p120, but not in Δ-βcat, in comparison with VEC-null cells. The quantification of receptor phosphorylation data from three experiments ± SD is shown in A on the right. The values represent the ratio between the phosphorylated and total amount of VEGFR-2 and are normalized to the ratio calculated in untreated VEC-positive cells. The peak of VEGFR-2 phosphorylation at 5 min is similar in VEC-null and Δ-βcat, but lower in Δ-p120. At longer stimulation (30 min), the level of phosphorylation of VEGFR-2 in Δ-p120 was comparable to VEC-positive cells. Incubation of VEC-positive cell extract with nonimmune (NI) rabbit immunoglobulin (matched with VEGFR-2 antibody used for IP) did not precipitate bands corresponding to either VEGFR-2 or VE-cadherin, last lane from the left (IP NI). (B) VE-cadherin mutants modulate endothelial growth induced by VEGF. VEC-null and Δ-βcat had comparable effects and were the most permissive mutations in terms of cell proliferation (>160% increase over VEC-positive cells). Mutations that affected binding of p120 (Δ-p120) allowed cell proliferation, but to a lower extent (60% increase over stimulation of VEC-positive cells). Proliferation was measured as BrdU incorporation as described in the legend to Fig. 1. Mean ± SD of three independent experiments, each in duplicate, is shown.
Figure 7.
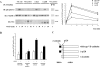
The absence of β-catenin enhances VEGF-induced phosphorylation of VEGFR-2 and cell proliferation. Endothelial cells derived from β-catenin–null embryos (β-cat null) did not express β-catenin in comparison with cells obtained from β-catenin–positive (β-cat positive) littermate animals. (A) By immunofluorescence analysis, VE-cadherin was expressed at a comparable level and was concentrated at cell–cell contacts in both β-catenin–null and –positive cells. Cell junctions were negative for β-catenin in null cells. Bars: (phase contrast) 100 μm; (VE-cadherin and β-catenin) 20 μm. (B) Immunoprecipitation (IP) of cell extracts with VE-cadherin antibodies followed by Western blot (IB) with anti–VE-cadherin (αVE-cadherin) or β-catenin (αβ-catenin) antibodies showed absence of the last protein in the complex. (C) The absence of β-catenin enhanced the extent and duration of VEGFR-2 phosphorylation in response to VEGF (80 ng/ml). IP with anti–VEGFR-2 and Western blot with antiphosphotyrosine and anti–VEGFR-2 antibodies. (D) VE-cadherin could be coimmunoprecipitated with VEGFR-2 only in β-positive cells after VEGF (80 ng/ml for 5 min). IP with anti–VEGFR-2 and Western blot with anti–VEGFR-2 and anti–VE-cadherin antibodies. (E) Confluent β-cat–null endothelial cells incorporated BrdU 2–2.5-fold more than β-cat–positive cells in response to stimulation with VEGF (80 ng/ml for 24 h). Incorporation of BrdU was measured and calculated (mean of three independent experiments ± SD) as in Fig. 1. Two independent pairs of both β-positive and β-null endothelial cells obtained from littermate embryos of different mothers have been tested with comparable results.
Figure 8.
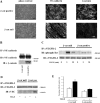
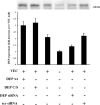
Expression of tyrosine phosphatase DEP-1 in VEC-positive and -negative cells and effect of transfection of wild-type and DEP-1 C/S constructs and DEP-1 RNAi. In VEC-positive endothelial cells (third lane), DEP-1 was 80–90% higher than in VEC null (fourth lane). In VEC-positive cells, transfection of wild-type (wt, first lane) or point-mutated (C/S, second lane) DEP-1 constructs resulted in increased expression of the protein (50–60% more than in control VEC positive). siRNA directed against DEP-1 (DEP siRNA) resulted in 35–40% inhibition of DEP expression (fifth lane). Scramble oligonucleotides (scr siRNA), used as control (100 nM as DEP siRNA oligonucleotide), were ineffective. Plus and minus indicate the presence or the absence of the protein indicated on the left. The columns report the mean of four experiments ± SD.
Figure 9.
Down-regulation of DEP-1 increases VEGFR-2 phosphorylation. Transfection of the dominant-negative DEP C/S mutant or DEP siRNA induced higher phosphorylation of VEGFR-2 upon VEGF (80 ng/ml for 5 min) than the respective controls, VEC positive, DEP wild type (DEP wt), and scramble siRNA (scr siRNA), respectively. Data were obtained by immunoprecipitation (IP) with anti–VEGFR-2 antibodies (αVEGFR-2) followed by Western blot (IB) with an antiphosphotyrosine antibody (αphosphoTyr). The graph was obtained by quantifying the gel bands and calculating the ratio of bands labeled with antiphosphotyrosine antibodies over total VEGFR-2. Each column represents the fold increase over unstimulated cells. Data are means ± SD of four experiments. Plus and minus indicate the presence or the absence of the protein indicated on the left.
Figure 10.
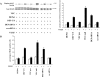
Inhibition of DEP-1 enhances phosphorylation of p44/42 MAP kinase and BrdU nuclear incorporation in endothelial cells. (A) Phosphorylation of p44/42 MAP kinase in response to VEGF (80 ng/ml for 10 min) is increased by 80–90% in VEC-positive cells expressing DEP C/S in comparison with control VEC positive. A similar increase is observed after DEP siRNA transfection of the cells. The graph represents the ratio between phosphorylated and total p44/42 MAP kinase as fold increase over untreated VEC-null cells. The values are means ± SD of four independent experiments. Plus and minus indicate the presence or the absence of the protein indicated on the left. (B) Nuclear incorporation of BrdU was stimulated by 80–100% in VEC-positive cells expressing C/S DEP or after DEP siRNA. BrdU incorporation was assayed as in Fig. 1. Data are means ± SD of four experiments.
Similar articles
- Impaired VE-cadherin/beta-catenin expression mediates endothelial cell degeneration in dilated cardiomyopathy.
Schäfer R, Abraham D, Paulus P, Blumer R, Grimm M, Wojta J, Aharinejad S. Schäfer R, et al. Circulation. 2003 Sep 30;108(13):1585-91. doi: 10.1161/01.CIR.0000091085.12422.19. Epub 2003 Sep 8. Circulation. 2003. PMID: 12963640 - HGF/NK4 inhibited VEGF-induced angiogenesis in in vitro cultured endothelial cells and in vivo rabbit model.
Nakabayashi M, Morishita R, Nakagami H, Kuba K, Matsumoto K, Nakamura T, Tano Y, Kaneda Y. Nakabayashi M, et al. Diabetologia. 2003 Jan;46(1):115-23. doi: 10.1007/s00125-002-0954-y. Epub 2002 Dec 6. Diabetologia. 2003. PMID: 12637990 - Regulation of VE-cadherin linkage to the cytoskeleton in endothelial cells exposed to fluid shear stress.
Ukropec JA, Hollinger MK, Woolkalis MJ. Ukropec JA, et al. Exp Cell Res. 2002 Feb 15;273(2):240-7. doi: 10.1006/excr.2001.5453. Exp Cell Res. 2002. PMID: 11822879 - Turn-off, drop-out: functional state switching of cadherins.
Lilien J, Balsamo J, Arregui C, Xu G. Lilien J, et al. Dev Dyn. 2002 May;224(1):18-29. doi: 10.1002/dvdy.10087. Dev Dyn. 2002. PMID: 11984870 Review. - Angiogenesis: the VE-cadherin switch.
Wallez Y, Vilgrain I, Huber P. Wallez Y, et al. Trends Cardiovasc Med. 2006 Feb;16(2):55-9. doi: 10.1016/j.tcm.2005.11.008. Trends Cardiovasc Med. 2006. PMID: 16473763 Review.
Cited by
- Fibroblast growth factor signaling potentiates VE-cadherin stability at adherens junctions by regulating SHP2.
Hatanaka K, Lanahan AA, Murakami M, Simons M. Hatanaka K, et al. PLoS One. 2012;7(5):e37600. doi: 10.1371/journal.pone.0037600. Epub 2012 May 22. PLoS One. 2012. PMID: 22629427 Free PMC article. - A VE-cadherin-PAR3-α-catenin complex regulates the Golgi localization and activity of cytosolic phospholipase A(2)α in endothelial cells.
Odell AF, Hollstein M, Ponnambalam S, Walker JH. Odell AF, et al. Mol Biol Cell. 2012 May;23(9):1783-96. doi: 10.1091/mbc.E11-08-0694. Epub 2012 Mar 7. Mol Biol Cell. 2012. PMID: 22398721 Free PMC article. - Adherens junction turnover: regulating adhesion through cadherin endocytosis, degradation, and recycling.
Kowalczyk AP, Nanes BA. Kowalczyk AP, et al. Subcell Biochem. 2012;60:197-222. doi: 10.1007/978-94-007-4186-7_9. Subcell Biochem. 2012. PMID: 22674073 Free PMC article. Review. - Angiogenic sprouting requires the fine tuning of endothelial cell cohesion by the Raf-1/Rok-α complex.
Wimmer R, Cseh B, Maier B, Scherrer K, Baccarini M. Wimmer R, et al. Dev Cell. 2012 Jan 17;22(1):158-71. doi: 10.1016/j.devcel.2011.11.012. Epub 2011 Dec 29. Dev Cell. 2012. PMID: 22209329 Free PMC article. - Transmembrane protein ESDN promotes endothelial VEGF signaling and regulates angiogenesis.
Nie L, Guo X, Esmailzadeh L, Zhang J, Asadi A, Collinge M, Li X, Kim JD, Woolls M, Jin SW, Dubrac A, Eichmann A, Simons M, Bender JR, Sadeghi MM. Nie L, et al. J Clin Invest. 2013 Dec;123(12):5082-97. doi: 10.1172/JCI67752. Epub 2013 Nov 1. J Clin Invest. 2013. PMID: 24177422 Free PMC article.
References
- Balconi, G., R. Spagnuolo, and E. Dejana. 2000. Development of endothelial cell lines from embryonic stem cells: a tool for studying genetically manipulated endothelial cells in vitro. Arterioscler. Thromb. Vasc. Biol. 20:1443–1451. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous