Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins - PubMed (original) (raw)
Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins
Susumu Tomita et al. J Cell Biol. 2003.
Abstract
Functional expression of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors in cerebellar granule cells requires stargazin, a member of a large family of four-pass transmembrane proteins. Here, we define a family of transmembrane AMPA receptor regulatory proteins (TARPs), which comprise stargazin, gamma-3, gamma-4, and gamma-8, but not related proteins, that mediate surface expression of AMPA receptors. TARPs exhibit discrete and complementary patterns of expression in both neurons and glia in the developing and mature central nervous system. In brain regions that express multiple isoforms, such as cerebral cortex, TARP-AMPA receptor complexes are strictly segregated, suggesting distinct roles for TARP isoforms. TARPs interact with AMPA receptors at the postsynaptic density, and surface expression of mature AMPA receptors requires a TARP. These studies indicate a general role for TARPs in controlling synaptic AMPA receptors throughout the central nervous system.
Figures
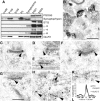
Figure 1.
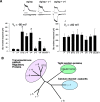
Functional identification of a family of TRAPs. (A) Cerebellar granule cells from stargazer mutant mice were transfected with γ-1, stargazin (STG), γ-3, γ-4, γ-5, γ-8, or claudin-1, and whole cell responses to glutamate were recorded. The bar between traces represents 3-s applications of glutamate (100 μM) and cyclothiazide (100 μM). Calibration bars: 50 pA, and 1 s TARP family proteins. stargazin (STG), γ-3, γ-4, and γ-8 restore AMPA-type glutamate-evoked responses (recorded at –80 mV) in stg/stg cerebellar granule cells (n = 5, P < 0.01), but γ-1, γ-5, and claudin-1 do not. The NMDA-type glutamate-evoked responses (recorded at +60 mV) were not significantly altered by any of the transfections. Bottom bar graphs summarize data from these neuronal transfection experiments. (B) Phylogenetic tree shows relationship of TARPs to other related four pass transmembrane proteins.
Figure 2.
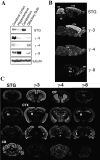
Differential distribution of TRAPs in adult brain. (A) Immunoblotting shows highest levels of stargazin (γ-2) in cerebellum, γ-3 in cerebral cortex, γ-4 in olfactory bulb, and γ-8 in hippocampus. In situ hybridization on sagittal (B) and coronal (C) sections show that stargazin mRNA is concentrated in cerebellum and also occurs in cerebral cortex (CTX), hippocampus (H), and facial nerve nucleus (VII). γ-3 is restricted to forebrain and occurs at highest levels in cortex, hippocampus, and olfactory tubercle (TU) and at lower levels in caudate putamen. γ-4 occurs diffusely throughout the adult brain but appears distinctly enriched in caudate putamen (CP) and habenula (HA). γ-8 mRNA is highly enriched in hippocampus and modestly expressed in cortex and olfactory bulb (OB).
Figure 3.
γ-4 is expressed in glial cells in adult rat. In situ hybridization (A, B, E, and F) and immunocytochemistry (C, D, G, and H) show that γ-4 but not stargazin (STG) is expressed in glial cells. (A) In cerebellum, stargazin mRNA is expressed in granule cells (GC), Purkinje cells (PC; arrowheads), and in interneurons (arrows) in molecular layer (ML). (B) In contrast, γ-4 mRNA occurs in a diffuse band along the Purkinje cell layer that reflects expression in Bergmann glia (BG) as indicated in C by immunostaining of their apical processes in the molecular layer (arrows). Scattered cells in cerebellum showing γ-4 mRNA expression (B, arrows) are glia, since immunostaining shows their fibrous star-shaped morphology (D, arrows). (E) In neocortex, stargazin mRNA is expressed in neurons of cerebral cortex (Cx), granule cells of dentate gyrus (DG), and pyramidal cells of the hippocampus (CA). Stargazin is also expressed in interneurons of hilus of dentate gyrus (arrows) and in stratum oriens (arrowheads) and stratum radiatum. (F) In contrast, γ-4 mRNA occurs in scattered cells throughout cerebral cortex (Cx), corpus callosum (CC), and hippocampus (arrows) that resemble glia as detected by immunocytochemistry (G and H).
Figure 4.
Developmental switch in neuronal expression of TARPs. Immunoblotting shows that γ-4 protein levels peak in rat pups at postnatal day 6 (P6) and decline thereafter in cerebral cortex. In contrast, expression of stargazin (STG), γ-3, and γ-8 appear later and progressively increase during animal maturation. In situ hybridization at embryonic day 16 (E16) shows that γ-4 mRNA but not that of other TARPs occurs at extremely high levels throughout the nervous system in cortical plate (CP), olfactory epithelium (OE), dorsal root ganglia (DRG), and spinal cord (SC). Some γ-4 mRNA also occurs in peripheral tissues such as the intestine (IN).
Figure 5.
TARPs cluster selectively at excitatory synapses that contain AMPA receptors. An antibody (pan-TARP) that reacts with all TARPs was used to stain cultured hippocampal cultures. (First row) Immunofluorescence for TARPs occurs at punctate sites (arrows) along the dendrites that closely colocalize with the GluR2 subunit of the AMPA receptor (AMPAR). (Second row) Virtually all TARP puncta colocalize with PSD-95 (arrows), but some PSD-95 puncta lack TARP immunofluorescence (arrowheads). (Third row) Almost all TARP puncta colocalize with NMDAR1 (arrows), but some NMDAR1 puncta lack TARP immunofluorescence. (Fourth row) TARPs show no overlap with the GABAergic marker GAD65. Preabsorbing the antibody with antigen (10 μM) blocks labeling (small boxes in second row).
Figure 6.
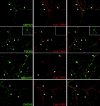
TARPs colocalize with AMPA receptors at excitatory synapses. (A) Western blotting shows that AMPA receptor trafficking proteins are all highly enriched in PSD fractions. By contrast, synaptophysin, which is also enriched in the crude synaptosomes is extracted with 0.5% Triton X-100 and does not occur in PSD fractions. (B) Pre-embedding DAB immunoperoxidase labeling for pan-TARP in the CA1 stratum radiatum of the hippocampus. Labeling is concentrated in dendritic shafts and associated postsynaptic spines. Note the dense concentration of labeling at postsynaptic densities (arrowheads). Patches of labeling also are evident in the spine and dendrite cytoplasm. (C–L) Double immunogold labeling for pan-TARP (5 nm gold) and GluR2/3 (10 nm gold) in the hippocampus. (C, D, and H) CA1 stratum radiatum. (F and G) Hilus. (E and I) CA3 stratum lucidum. (J) Molecular layer of the dentate gyrus. (K) CA1 stratum oriens. Black arrowheads indicate 5-nm gold particles that is associated closely with 10 nm gold particles. In C and I, one of the 5-nm gold particles overlaps a 10-nm gold particle. Close associations of 5- and 10-nm gold particles are more common at synapses (C–H) than in dendrite cytoplasm (I–K). H shows a very oblique synapse; 5 and 10-nm gold particles are associated in a presumptive vesicle fused to the lateral wall of the postsynaptic spine. Bars: (B) 500 nm; (in G, for C–K) 200 nm. (L) Distribution of gold particles (y axis) in the perpendicular (axodendritic) axis of synapses. Zero represents the postsynaptic membrane; + is toward presynaptic and – is toward postsynaptic. Note how most gold for TARPs and GluR2/3 is concentrated at the postsynaptic membrane and within the postsynaptic density.
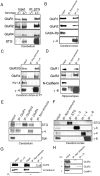
Figure 7.
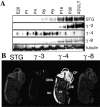
TARPs interact specifically with AMPA receptors in brain extracts. (A) AMPA receptor subunits GluR1, GluR2, and GluR4 coimmunoprecipitate with stargazin (STG) in brain extracts from +/stg mice (+/−). In extracts from stg/stg mice (−/−), the antistargazin antibody does not immunoprecipitate GluR1, GluR2, or GluR4. (B–D) AMPA receptor subunits also coimmunoprecipitate with γ-3, γ-4, and γ-8 in extracts from adult cerebral cortex, postnatal day 7 cerebral cortex, and hippocampus, respectively. As control, other transmembrane proteins such as GABAARβ, N-cadherin, and Kv1.4 did not coimmunoprecipitate. (E) Subunit specificity for TARP interactions in cerebellum. Stargazin (STG) and γ-4 coimmunoprecipitate with GluR1 and GluR4, but only stargazin coimmunoprecipitates with GluR2/3 in cerebellum. (F) TARP isoforms are strictly segregated, since they do not coimmunoprecipitate with one another in cerebral cortex. (G) Surface GluR2 coimmunoprecipitates with γ-3. Primary cerebrocortical cultures were incubated with a membrane impermeable cross-linker (CL), and after SDS solubilization extracts were immunoprecipitated for γ-3; GluR2 coprecipitates with γ-3 in a cross-linker–dependent fashion, whereas another synaptic transmembrane protein, N-cadherin, does not. H, GluR2 coimmunoprecipitated with γ-3 in chemical cross-linked PSD fractions from brain, whereas another PSD protein, CaMKII, does not.
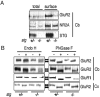
Figure 8.
Stargazin enhances mature glycosylation and surface expression of an AMPA receptor subunit protein. (A) Cerebellar (Cb) cultures from +/stg(+/−) or stg/stg(−/−) were biotinylated, solubilized, and precipitated with streptavidin-agarose. Surface expression of the AMPA receptor subunit GluR2 is dramatically decreased in stg/stg cultures, whereas NMDAR subunits NR2A are not significantly altered. (B) In cerebellum of stargazer mouse (−/−), a large fraction of GluR2 remains immature and sensitive to EndoH glycosidase. By contrast, GluR2 in stargazer cerebral cortex (Cx) is mature and resistant to EndoH. In all cases, GluR2 glycosylation is removed by the nonspecific N-glycosidase, PNGaseF. Total levels of GluR2 were consistently decreased by ∼10–20% in cerebellum from stg/stg mice. Note that two independent examples from stargazer cerebellum are presented.
Similar articles
- TARPs gamma-2 and gamma-7 are essential for AMPA receptor expression in the cerebellum.
Yamazaki M, Fukaya M, Hashimoto K, Yamasaki M, Tsujita M, Itakura M, Abe M, Natsume R, Takahashi M, Kano M, Sakimura K, Watanabe M. Yamazaki M, et al. Eur J Neurosci. 2010 Jun;31(12):2204-20. doi: 10.1111/j.1460-9568.2010.07254.x. Epub 2010 Jun 7. Eur J Neurosci. 2010. PMID: 20529126 - Dynamic interaction of stargazin-like TARPs with cycling AMPA receptors at synapses.
Tomita S, Fukata M, Nicoll RA, Bredt DS. Tomita S, et al. Science. 2004 Mar 5;303(5663):1508-11. doi: 10.1126/science.1090262. Science. 2004. PMID: 15001777 - Two families of TARP isoforms that have distinct effects on the kinetic properties of AMPA receptors and synaptic currents.
Cho CH, St-Gelais F, Zhang W, Tomita S, Howe JR. Cho CH, et al. Neuron. 2007 Sep 20;55(6):890-904. doi: 10.1016/j.neuron.2007.08.024. Neuron. 2007. PMID: 17880893 - Auxiliary subunits assist AMPA-type glutamate receptors.
Nicoll RA, Tomita S, Bredt DS. Nicoll RA, et al. Science. 2006 Mar 3;311(5765):1253-6. doi: 10.1126/science.1123339. Science. 2006. PMID: 16513974 Review. - Regulation of AMPA receptors by transmembrane accessory proteins.
Díaz E. Díaz E. Eur J Neurosci. 2010 Jul;32(2):261-8. doi: 10.1111/j.1460-9568.2010.07357.x. Eur J Neurosci. 2010. PMID: 20946114 Review.
Cited by
- Neto-α Controls Synapse Organization and Homeostasis at the Drosophila Neuromuscular Junction.
Han TH, Vicidomini R, Ramos CI, Wang Q, Nguyen P, Jarnik M, Lee CH, Stawarski M, Hernandez RX, Macleod GT, Serpe M. Han TH, et al. Cell Rep. 2020 Jul 7;32(1):107866. doi: 10.1016/j.celrep.2020.107866. Cell Rep. 2020. PMID: 32640231 Free PMC article. - Cornichons modify channel properties of recombinant and glial AMPA receptors.
Coombs ID, Soto D, Zonouzi M, Renzi M, Shelley C, Farrant M, Cull-Candy SG. Coombs ID, et al. J Neurosci. 2012 Jul 18;32(29):9796-804. doi: 10.1523/JNEUROSCI.0345-12.2012. J Neurosci. 2012. PMID: 22815494 Free PMC article. - TARP subtypes differentially and dose-dependently control synaptic AMPA receptor gating.
Milstein AD, Zhou W, Karimzadegan S, Bredt DS, Nicoll RA. Milstein AD, et al. Neuron. 2007 Sep 20;55(6):905-18. doi: 10.1016/j.neuron.2007.08.022. Neuron. 2007. PMID: 17880894 Free PMC article. - Altered GABAA Receptor Expression in the Primary Somatosensory Cortex of a Mouse Model of Genetic Absence Epilepsy.
Hassan M, Adotevi NK, Leitch B. Hassan M, et al. Int J Mol Sci. 2022 Dec 10;23(24):15685. doi: 10.3390/ijms232415685. Int J Mol Sci. 2022. PMID: 36555327 Free PMC article. - AMPA receptor synaptic targeting regulated by stargazin interactions with the Golgi-resident PDZ protein nPIST.
Cuadra AE, Kuo SH, Kawasaki Y, Bredt DS, Chetkovich DM. Cuadra AE, et al. J Neurosci. 2004 Aug 25;24(34):7491-502. doi: 10.1523/JNEUROSCI.1255-04.2004. J Neurosci. 2004. PMID: 15329396 Free PMC article.
References
- Burgess, D.L., L.A. Gefrides, P.J. Foreman, and J.L. Noebels. 2001. A cluster of three novel Ca2+ channel gamma subunit genes on chromosome 19q13.4: evolution and expression profile of the gamma subunit gene family. Genomics. 71:339–350. - PubMed
- Chen, L., D.M. Chetkovich, R. Petralia, N. Sweeney, Y. Kawaski, R. Wenthold, D.S. Bredt, and R.A. Nicoll. 2000. Stargazin mediates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 408:936–943. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases