Altered skeletal muscle phenotypes in calcineurin Aalpha and Abeta gene-targeted mice - PubMed (original) (raw)
Altered skeletal muscle phenotypes in calcineurin Aalpha and Abeta gene-targeted mice
Stephanie A Parsons et al. Mol Cell Biol. 2003 Jun.
Abstract
Calcineurin is a calcium-regulated serine-threonine protein phosphatase that controls developmental and inducible biological responses in diverse cell types, in part through activation of the transcription factor nuclear factor of activated T cells (NFAT). In skeletal muscle, calcineurin has been implicated in the regulation of myoblast differentiation, hypertrophy of mature myofibers, and fiber type switching in response to alterations in intracellular calcium concentration. However, considerable disagreement persists about the functional role of calcineurin signaling in each of these processes. Here we evaluated the molecular phenotypes of skeletal muscle from both calcineurin Aalpha and calcineurin Abeta gene-targeted mice. Calcineurin Aalpha was observed to be the predominant catalytic isoform expressed in nearly all skeletal muscles examined. Neither calcineurin Aalpha or Abeta null mice showed any gross growth-related alterations in skeletal muscle, nor was fiber size or number altered in glycolytic/fast muscle types. In contrast, both calcineurin Aalpha and Abeta gene-targeted mice demonstrated an alteration in myofiber number in the soleus, an oxidative/slow-type muscle. More significantly, calcineurin Aalpha and Abeta gene-targeted mice showed a dramatic down-regulation in the oxidative/slow fiber type program in multiple muscles (both slow and fast). Associated with this observation, NFAT-luciferase reporter transgenic mice showed significantly greater activity in slow fiber-containing muscles than in fast. However, only calcineurin Aalpha null mice showed a defect in NFAT nuclear occupancy or NFAT-luciferase transgene activity in vivo. Collectively, our results suggest that calcineurin signaling plays a critical role in regulating skeletal muscle fiber type switching but not hypertrophy. Our results also suggest that fiber type switching occurs through an NFAT-independent mechanism.
Figures
FIG. 1.
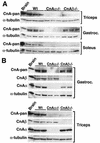
Western blots of calcineurin protein expression in mouse skeletal muscles. (A) Western blots of total calcineurin A expression in triceps, gastrocnemius, and soleus from wild-type (Wt), calcineurin Aα−/−, and _Aβ_−/− mice. α-Tubulin was included as a loading control. (B) Isoform-specific calcineurin A Western blots of protein extracts from gastrocnemius and triceps from wild-type or calcineurin A gene-targeted mice.
FIG. 2.
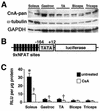
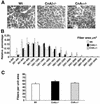
Calcineurin expression and activity between fast and slow muscles. (A) Western blot of total calcineurin A protein content between oxidative/slow (soleus) and glycolytic/fast (gastrocnemius, tibialis anterior [TA], biceps, and triceps) muscles. α-Tubulin and GAPDH are shown as loading controls. (B) Schematic of the transgene construct that was used to make NFAT-dependent reporter mice. (C) Luciferase activity normalized as relative light units (RLU) per microgram of protein from fast and slow muscles taken from untreated transgenic mice (n = 5 mice) or CsA-treated mice (n = 8 mice) (asterisk indicates P < 0.05 versus any of the four untreated fast muscles, and daggers indicate P < 0.05 versus untreated muscles).
FIG. 3.
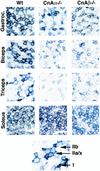
NADH-tetrazolium staining of various skeletal muscles from wild-type, calcineurin Aα−/−, and _Aβ_−/− mice. The darkest blue fibers are the most oxidative (type I), the lighter blue fibers are intermediate oxidative/glycolytic (type IIa/x), and the unstained fibers are glycolytic (type IIb).
FIG. 4.
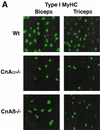
Immunostaining for MyHC expression in muscles from wild-type, calcineurin Aα−/−, and _Aβ_−/− mice. (A) Type I (slow) MyHC expression is noticeably reduced in biceps and triceps from both calcineurin Aα−/− and _Aβ_−/− mice (green stain is fluorescein isothiocyanate-conjugated secondary antibody to detect type I MyHC). (B) Type I (slow) and type II (fast) MyHC expression in soleus muscle from calcineurin Aα−/− and _Aβ_−/− mice.
FIG. 4.
Immunostaining for MyHC expression in muscles from wild-type, calcineurin Aα−/−, and _Aβ_−/− mice. (A) Type I (slow) MyHC expression is noticeably reduced in biceps and triceps from both calcineurin Aα−/− and _Aβ_−/− mice (green stain is fluorescein isothiocyanate-conjugated secondary antibody to detect type I MyHC). (B) Type I (slow) and type II (fast) MyHC expression in soleus muscle from calcineurin Aα−/− and _Aβ_−/− mice.
FIG. 5.
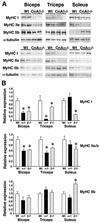
Western analysis of MyHC protein expression. (A) Biceps, triceps, and soleus from wild-type, calcineurin Aα−/−, and _Aβ_−/− mice were subjected to Western blotting to detect MyHC I, IIa, and IIb isoform expression. (B) Quantitation of MyHC Western blots (from four individual mice). Asterisks indicate P < 0.05 versus wild-type mice.
FIG. 6.
Histological analysis of EDL from wild-type, calcineurin Aα−/−, and _Aβ_−/− mice. (A) Representative sections of wheat germ agglutinin-TRITC-labeled EDL muscle. (B) Fiber areas in four mice each were measured from wild-type, calcineurin Aα−/−, and _Aβ_−/− mice. Approximately 300 fibers were measured per muscle section and grouped by size in 200-μm2 increments. (C) Average number of fibers per unit of area (100,000 μm2). No values were significantly different.
FIG. 7.
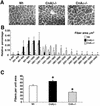
Histological analysis of soleus from wild-type, calcineurin Aα−/−, and _Aβ_−/− mice. (A) Representative sections of wheat germ agglutinin-TRITC-labeled soleus muscle. (B) Fiber areas in four mice each were measured from wild-type, calcineurin Aα−/−, and _Aβ_−/− mice. Approximately 300 fibers were measured per muscle section and grouped by size in 200-μm2 increments. Asterisks indicate P < 0.05 for wild-type versus calcineurin Aβ−/− mice, and daggers indicate P < 0.05 for wild-type versus calcineurin Aα−/− mice. (C) Average number of fibers per unit of area (100,000 μm2). Asterisks indicate P < 0.05 versus wild type.
FIG. 8.
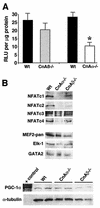
(A) Relative luciferase activity (in relative light units [RLU] per microgram of protein) from soleus muscle from mice containing the NFAT-luciferase reporter crossed into either calcineurin Aα−/− (n = 4) or _Aβ_−/− (n = 5) mice compared with that of littermate wild-type controls (n = 6). Asterisks indicate P < 0.05 versus wild type. (B) Western blots of nuclear proteins from skeletal muscle of wild-type, calcineurin Aα−/−, and _Aβ_−/− mice (n = 3 mice per nuclear protein preparation per experiment). Western blotting for NFATc1, -c2, -c3, and -c4 revealed a significant reduction in nuclear content in the absence of calcineurin Aα, but not Aβ. No change in total MEF2, Elk-1, or GATA2 was detected from any of the nuclear extracts or in PGC-1α from soleus whole-cell protein extracts.
Similar articles
- Genetic loss of calcineurin blocks mechanical overload-induced skeletal muscle fiber type switching but not hypertrophy.
Parsons SA, Millay DP, Wilkins BJ, Bueno OF, Tsika GL, Neilson JR, Liberatore CM, Yutzey KE, Crabtree GR, Tsika RW, Molkentin JD. Parsons SA, et al. J Biol Chem. 2004 Jun 18;279(25):26192-200. doi: 10.1074/jbc.M313800200. Epub 2004 Apr 13. J Biol Chem. 2004. PMID: 15082723 - The calcineurin-NFAT pathway and muscle fiber-type gene expression.
Swoap SJ, Hunter RB, Stevenson EJ, Felton HM, Kansagra NV, Lang JM, Esser KA, Kandarian SC. Swoap SJ, et al. Am J Physiol Cell Physiol. 2000 Oct;279(4):C915-24. doi: 10.1152/ajpcell.2000.279.4.C915. Am J Physiol Cell Physiol. 2000. PMID: 11003571 - Effects of sarcolipin deletion on skeletal muscle adaptive responses to functional overload and unload.
Fajardo VA, Rietze BA, Chambers PJ, Bellissimo C, Bombardier E, Quadrilatero J, Tupling AR. Fajardo VA, et al. Am J Physiol Cell Physiol. 2017 Aug 1;313(2):C154-C161. doi: 10.1152/ajpcell.00291.2016. Epub 2017 Jun 7. Am J Physiol Cell Physiol. 2017. PMID: 28592414 - The Ca2+/Calmodulin-dependent Calcineurin/NFAT Signaling Pathway in the Pathogenesis of Insulin Resistance in Skeletal Muscle.
Danowska M, Strączkowski M. Danowska M, et al. Exp Clin Endocrinol Diabetes. 2023 Nov;131(11):589-594. doi: 10.1055/a-2174-7958. Epub 2023 Oct 24. Exp Clin Endocrinol Diabetes. 2023. PMID: 37875146 Review. - Calcineurin: a poorly understood regulator of muscle mass.
Hudson MB, Price SR. Hudson MB, et al. Int J Biochem Cell Biol. 2013 Oct;45(10):2173-8. doi: 10.1016/j.biocel.2013.06.029. Epub 2013 Jul 6. Int J Biochem Cell Biol. 2013. PMID: 23838168 Free PMC article. Review.
Cited by
- Modulatory calcineurin-interacting proteins 1 and 2 function as calcineurin facilitators in vivo.
Sanna B, Brandt EB, Kaiser RA, Pfluger P, Witt SA, Kimball TR, van Rooij E, De Windt LJ, Rothenberg ME, Tschop MH, Benoit SC, Molkentin JD. Sanna B, et al. Proc Natl Acad Sci U S A. 2006 May 9;103(19):7327-32. doi: 10.1073/pnas.0509340103. Epub 2006 Apr 28. Proc Natl Acad Sci U S A. 2006. PMID: 16648267 Free PMC article. - Exercise, PGC-1alpha, and metabolic adaptation in skeletal muscle.
Yan Z. Yan Z. Appl Physiol Nutr Metab. 2009 Jun;34(3):424-7. doi: 10.1139/H09-030. Appl Physiol Nutr Metab. 2009. PMID: 19448709 Free PMC article. Review. - Calcineurin plays a modulatory role in loading-induced regulation of type I myosin heavy chain gene expression in slow skeletal muscle.
Pandorf CE, Jiang WH, Qin AX, Bodell PW, Baldwin KM, Haddad F. Pandorf CE, et al. Am J Physiol Regul Integr Comp Physiol. 2009 Oct;297(4):R1037-48. doi: 10.1152/ajpregu.00349.2009. Epub 2009 Aug 5. Am J Physiol Regul Integr Comp Physiol. 2009. PMID: 19657098 Free PMC article. - The Ca(V) 1.2 Ca(2+) channel is expressed in sarcolemma of type I and IIa myofibers of adult skeletal muscle.
Jeftinija DM, Wang QB, Hebert SL, Norris CM, Yan Z, Rich MM, Kraner SD. Jeftinija DM, et al. Muscle Nerve. 2007 Oct;36(4):482-90. doi: 10.1002/mus.20842. Muscle Nerve. 2007. PMID: 17636479 Free PMC article. - Cooperative synergy between NFAT and MyoD regulates myogenin expression and myogenesis.
Armand AS, Bourajjaj M, Martínez-Martínez S, el Azzouzi H, da Costa Martins PA, Hatzis P, Seidler T, Redondo JM, De Windt LJ. Armand AS, et al. J Biol Chem. 2008 Oct 24;283(43):29004-10. doi: 10.1074/jbc.M801297200. Epub 2008 Aug 1. J Biol Chem. 2008. PMID: 18676376 Free PMC article.
References
- Allen, D. L., and L. A. Leinwand. 2002. Intracellular calcium and myosin isoform transitions. Calcineurin and CAM kinase pathways regulate preferential activation of the IIa myosin heavy chain promoter. J. Biol. Chem. 277:45323-45330. - PubMed
- Barton-Davis, E. R., W. A. LaFramboise, and M. J. Kushmerick. 1996. Activity-dependent induction of slow myosin gene expression in isolated fast-twitch mouse muscle. Am. J. Physiol. 271:C1409-C1414. - PubMed
- Bigard, X., H. Sanchez, J. Zoll, P. Mateo, V. Rousseau, V. Veksler, and R. Ventura-Clapier. 2000. Calcineurin co-regulates contractile and metabolic components of slow muscle phenotype. J. Biol. Chem. 275:19653-19660. - PubMed
- Bodine, S. C., T. N. Stitt, M. Gonzalez, W. O. Kline, G. L. Stover, R. Bauerlein, E. Zlotchenko, A. Scrimgeour, J. C. Lawrence, D. J. Glass, and G. D. Yancopoulos. 2001. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 3:1014-1019. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 HL010336/HL/NHLBI NIH HHS/United States
- T32 HL007382/HL/NHLBI NIH HHS/United States
- 5T32 HL07382/HL/NHLBI NIH HHS/United States
- F32HL10336/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases