Mitochondrial uncoupling protein-2 protects the immature brain from excitotoxic neuronal death - PubMed (original) (raw)
Mitochondrial uncoupling protein-2 protects the immature brain from excitotoxic neuronal death
Patrick G Sullivan et al. Ann Neurol. 2003 Jun.
Abstract
Excitotoxic cell death is the fundamental process responsible for many human neurodegenerative disorders, yet the basic mechanisms involved are not fully understood. Here, we exploited the fact that the immature brain is remarkably resistant to seizure-induced excitotoxic cell death and examined the underlying protective mechanisms. We found that, unlike in the adult, seizures do not increase the formation of reactive oxygen species or result in mitochondrial dysfunction in neonatal brain, because of high levels of the mitochondrial uncoupling protein (UCP2). UCP2 expression and function were basally increased in neonatal brain by the fat-rich diet of maternal milk, and substituting a low-fat diet reduced UCP2, restored mitochondrial coupling, and permitted seizure-induced neuronal injury. Thus, modulation of UCP2 expression and function by dietary fat protects neonatal neurons from excitotoxicity by preventing mitochondrial dysfunction. This mechanism offers novel neuroprotective strategies for individuals, greater than 1% of the world's population, who are affected by seizures.
Figures
Fig 1
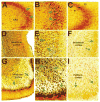
Neuronal injury in seizure-sensitive limbic regions of mature and postnatal day 10 (P10) rats after systemic administration of kainic acid., (A, B) Hippocampal CA3 from adult rats demonstrates neurons with silver affinity within the pyramidal cell layer (green arrows), indicating their injury.,,,, Such cells are not found in the P10 rat CA3 (C) including the pyramidal (sp), radiatum (sr), or oriens (so) cell layers. (D, E) Low and higher magnification views of the perirhinal cortex of mature rat show excitotoxicity in both deep and superficial layers of this seizure-vulnerable limbic region, whereas the corresponding region from a P10 rat (F) is free of silver-stained cells. (G–I) The piriform cortex, a highly seizure-vulnerable region, demonstrates seizure-injured neurons in adult (G, H) but not immature (I) brain.
Fig 2
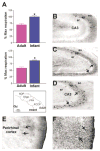
Uncoupling protein (UCP) function and UCP2 expression in seizure-vulnerable limbic brain regions of immature (P10) compared with adult rat. (A) Mitochondrial fractions from hippocampus (top) and perirhinal/piriform cortex (bottom) of the P10 rat are highly uncoupled on exposure to the UCP-activating free fatty acid (FFA) palmitate, when respiration is compared with the maximum respiration induced by the chemical uncoupler FCCP (bottom inset). Adult hippocampal and perirhinal/piriform cortex mitochondria respond modestly to FFA (indicating little UCP available for activation), whereas mitochondria from immature rats are completely uncoupled in the presence of palmitic acid (bars represent group means ± SEM; asterisk indicates p < 0.02). (B) When brain sections from immature and adult rats are processed for UCP2 immunocytochemistry, fewer UCP2-immunoreactive neurons (arrows) are evident in adult CA3 (8.7 ± 0.9) compared with the corresponding region of the P10 rat hippocampus (17.6 ± 0.6; C, D). (E, F) Compared with the rare UCP2-immunoreactive neurons in adult perirhinal cortex (E, arrows), dense staining is found in the limbic cortex of immature rats (F, arrows).
Fig 3
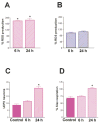
Differential effects of severe seizures on reactive oxygen species (ROS) production in mitochondria from immature and adult rat limbic neurons. (A) Evaluation of adult rat ROS production showed a significant increase at 6 and 24 hours after kainic acid administration, whereas (B) infant rat ROS production was not enhanced by these severe seizures. (C) Prolonged seizures significantly increased the number of UCP2-expressing neurons in the CA3 hippocampal region of mature rats. (D) Concomitantly, adult rat mitochondria demonstrated a significant increase in fatty acid–induced respiration (a measure of UCP function) 24 hours after the seizures. For all graphs, bars represent group means ± SEM; asterisks indicate p < 0.05).
Fig 4
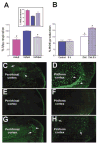
Reduction of dietary fat by substitution of an isocaloric, low-fat diet to immature rats reduces UCP function and promotes ROS production as well as seizure-induced excitotoxicity. (A) UCP function, measured as fatty acid–induced respiration, is significantly reduced in neonatal rats fed low-fat diet compared with maternal milk–fed littermates and resembles those in adult mitochondria. (inset) Basal ROS production in the presence of oligomycin (to maximize membrane potential) in isolated mitochondria from the group fed a low-fat diet are significantly increased compared with milk-fed littermates, approaching the basal levels found in adult mitochondria. (B) Energetic demand induced by severe seizures provokes striking increases in ROS production in UCP-suppressed (low-fat diet fed) neonatal rats, but not in those maintained on maternal milk. Bars represent means ± SEM; asterisks indicate p < 0.05). (C–H) Seizures provoke neuronal injury (visualized using Fluro-Jade) in several highly seizure-vulnerable regions of infant rats with suppressed UCP function. In adults, both perirhinal (C) and piriform (D) cortex demonstrated excitotoxic injury (arrows), whereas none was evident in the corresponding limbic regions of P10 rats on a “normal” high-fat diet (E, F). In striking contrast, excitotoxic injury occurred in perirhinal (G) and piriform (H) cortex of the low-fat diet fed infant rats (arrows).
Similar articles
- Peroxisome proliferator-activated receptors γ/mitochondrial uncoupling protein 2 signaling protects against seizure-induced neuronal cell death in the hippocampus following experimental status epilepticus.
Chuang YC, Lin TK, Huang HY, Chang WN, Liou CW, Chen SD, Chang AY, Chan SH. Chuang YC, et al. J Neuroinflammation. 2012 Jul 31;9:184. doi: 10.1186/1742-2094-9-184. J Neuroinflammation. 2012. PMID: 22849356 Free PMC article. - Kainic acid upregulates uncoupling protein-2 mRNA expression in the mouse brain.
Clavel S, Paradis E, Ricquier D, Richard D. Clavel S, et al. Neuroreport. 2003 Nov 14;14(16):2015-7. doi: 10.1097/00001756-200311140-00002. Neuroreport. 2003. PMID: 14600489 - Uncoupling protein 2 prevents neuronal death including that occurring during seizures: a mechanism for preconditioning.
Diano S, Matthews RT, Patrylo P, Yang L, Beal MF, Barnstable CJ, Horvath TL. Diano S, et al. Endocrinology. 2003 Nov;144(11):5014-21. doi: 10.1210/en.2003-0667. Epub 2003 Aug 21. Endocrinology. 2003. PMID: 12960023 - Mitochondrial uncoupling proteins in human physiology and disease.
Hagen T, Vidal-Puig A. Hagen T, et al. Minerva Med. 2002 Feb;93(1):41-57. Minerva Med. 2002. PMID: 11850613 Review. - Mitochondrial uncoupling proteins in the central nervous system.
Kim-Han JS, Dugan LL. Kim-Han JS, et al. Antioxid Redox Signal. 2005 Sep-Oct;7(9-10):1173-81. doi: 10.1089/ars.2005.7.1173. Antioxid Redox Signal. 2005. PMID: 16115020 Review.
Cited by
- UCP2 induced by natural birth regulates neuronal differentiation of the hippocampus and related adult behavior.
Simon-Areces J, Dietrich MO, Hermes G, Garcia-Segura LM, Arevalo MA, Horvath TL. Simon-Areces J, et al. PLoS One. 2012;7(8):e42911. doi: 10.1371/journal.pone.0042911. Epub 2012 Aug 8. PLoS One. 2012. PMID: 22905184 Free PMC article. - Traumatic brain injury and trichloroethylene exposure interact and produce functional, histological, and mitochondrial deficits.
Sauerbeck A, Hunter R, Bing G, Sullivan PG. Sauerbeck A, et al. Exp Neurol. 2012 Mar;234(1):85-94. doi: 10.1016/j.expneurol.2011.12.012. Epub 2011 Dec 20. Exp Neurol. 2012. PMID: 22201550 Free PMC article. - The role of mitochondrial uncoupling in the regulation of mitostasis after traumatic brain injury.
Hubbard WB, Velmurugan GV, Sullivan PG. Hubbard WB, et al. Neurochem Int. 2024 Mar;174:105680. doi: 10.1016/j.neuint.2024.105680. Epub 2024 Feb 3. Neurochem Int. 2024. PMID: 38311216 - Trichloroethylene induces dopaminergic neurodegeneration in Fisher 344 rats.
Liu M, Choi DY, Hunter RL, Pandya JD, Cass WA, Sullivan PG, Kim HC, Gash DM, Bing G. Liu M, et al. J Neurochem. 2010 Feb;112(3):773-83. doi: 10.1111/j.1471-4159.2009.06497.x. Epub 2009 Nov 17. J Neurochem. 2010. PMID: 19922440 Free PMC article. - Cyanide-induced death of dopaminergic cells is mediated by uncoupling protein-2 up-regulation and reduced Bcl-2 expression.
Zhang X, Li L, Zhang L, Borowitz JL, Isom GE. Zhang X, et al. Toxicol Appl Pharmacol. 2009 Jul 1;238(1):11-9. doi: 10.1016/j.taap.2009.03.020. Epub 2009 Apr 8. Toxicol Appl Pharmacol. 2009. PMID: 19361538 Free PMC article.
References
- Schwob JE, Fuller T, Price TL, Olney JW. Widespread patterns of neuronal damage following system or intracerebral injections of kainic acid: a histological study. Neuroscience. 1980;5:991–1014. - PubMed
- Ben-Ari Y, Tremblay E, Riche D, et al. Electrographic, clinical and pathological alterations following systemic administration of kainic acid, bicuculline or pentetrazole: metabolic mapping using the deoxyglucose method with special reference to the pathology of epilepsy. Neuroscience. 1981;6:1361–1391. - PubMed
- Holmes GL, Ben-Ari Y. Seizures in the developing brain: perhaps not so benign after all. Neuron. 1998;21:1231–1234. - PubMed
- Sutula TP, Pitkanen A. More evidence for seizure-induced neuron loss: is hippocampal sclerosis both cause and effect of epilepsy? Neurology. 2001;57:169–170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS035439-06/NS/NINDS NIH HHS/United States
- R01 NS028912-08/NS/NINDS NIH HHS/United States
- R01 NS035439/NS/NINDS NIH HHS/United States
- T32 NS007444/NS/NINDS NIH HHS/United States
- R01 NS028912-09/NS/NINDS NIH HHS/United States
- NS35439/NS/NINDS NIH HHS/United States
- R37 NS035439/NS/NINDS NIH HHS/United States
- NS07444/NS/NINDS NIH HHS/United States
- NS28912/NS/NINDS NIH HHS/United States
- R01 NS035439-05/NS/NINDS NIH HHS/United States
- R01 NS028912/NS/NINDS NIH HHS/United States
- NS32280/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources