Tetraspanins CD9 and CD81 function to prevent the fusion of mononuclear phagocytes - PubMed (original) (raw)
. 2003 Jun 9;161(5):945-56.
doi: 10.1083/jcb.200212031.
Isao Tachibana, Kenji Miyado, Masatoshi Kobayashi, Toru Miyazaki, Toshiki Funakoshi, Hiromi Kimura, Hiroyuki Yamane, Yoshiyuki Saito, Hiroyuki Goto, Tsutomu Yoneda, Mitsuhiro Yoshida, Toru Kumagai, Tadashi Osaki, Seiji Hayashi, Ichiro Kawase, Eisuke Mekada
Affiliations
- PMID: 12796480
- PMCID: PMC2172976
- DOI: 10.1083/jcb.200212031
Tetraspanins CD9 and CD81 function to prevent the fusion of mononuclear phagocytes
Yoshito Takeda et al. J Cell Biol. 2003.
Abstract
Tetraspanins CD9 and CD81 facilitate the fusion between gametes, myoblasts, or virus-infected cells. Here, we investigated the role of these tetraspanins in the fusion of mononuclear phagocytes. Expression of CD9 and CD81 and their complex formation with integrins were up-regulated when blood monocytes were cultured under normal conditions. Under fusogenic conditions in the presence of Con A, CD9 and CD81 up-regulation was inhibited, and their complex formation with integrins was down-regulated. Anti-CD9 and -CD81 antibodies, which were previously shown to inhibit the fusion of gametes, myoblasts, and virus-infected cells, unexpectedly promoted the fusion of monocytes and alveolar macrophages. However, these effects were not due to altered cell adhesion, aggregation, or cytokine production. When stimulated in vitro or in vivo, alveolar macrophages and bone marrow cells of CD9- and CD81-null mice formed larger numbers of multinucleated cells than those of wild-type mice. Finally, CD9/CD81 double-null mice spontaneously developed multinucleated giant cells in the lung and showed enhanced osteoclastogenesis in the bone. These results suggest that CD9 and CD81 coordinately prevent the fusion of mononuclear phagocytes.
Figures
Figure 1.
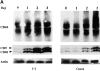
Con A modulates tetraspanin levels and integrin–tetraspanin complex formation in monocytes. (A) Blood monocytes were cultured in the absence (left) or presence (right) of 10 μg/ml Con A. After the indicated number of days, the cells were lysed with Brij99 lysis buffer. Whole-cell lysates containing equal amounts of protein were separated by SDS-PAGE and transferred to an Immobilon-P membrane. The membranes were blotted with anti-CD63 (AHN-16), anti-CD9 (MM2/57) plus anti-CD81 (M38), or anti-actin (C4) mAb. (B) Monocytes were lysed at d 0 or at d 3 in the absence or presence of Con A. Immunoprecipitations were performed with anti-β1 integrin (A-1A5), anti-β2 integrin (IB4), anti-CD9 (BU16), or anti-CD81 (M38) mAb. Immunoprecipitated proteins were electrophoresed, transferred to membranes, and probed with anti-CD9 or -CD81 mAb (left). To confirm the presence of comparable amounts of each protein, whole-cell lysates were blotted with anti-CD9, anti-CD81, anti-β1 (A-1A5), anti-β2 (MEM48), or anti-actin mAb (right).
Figure 1.
Con A modulates tetraspanin levels and integrin–tetraspanin complex formation in monocytes. (A) Blood monocytes were cultured in the absence (left) or presence (right) of 10 μg/ml Con A. After the indicated number of days, the cells were lysed with Brij99 lysis buffer. Whole-cell lysates containing equal amounts of protein were separated by SDS-PAGE and transferred to an Immobilon-P membrane. The membranes were blotted with anti-CD63 (AHN-16), anti-CD9 (MM2/57) plus anti-CD81 (M38), or anti-actin (C4) mAb. (B) Monocytes were lysed at d 0 or at d 3 in the absence or presence of Con A. Immunoprecipitations were performed with anti-β1 integrin (A-1A5), anti-β2 integrin (IB4), anti-CD9 (BU16), or anti-CD81 (M38) mAb. Immunoprecipitated proteins were electrophoresed, transferred to membranes, and probed with anti-CD9 or -CD81 mAb (left). To confirm the presence of comparable amounts of each protein, whole-cell lysates were blotted with anti-CD9, anti-CD81, anti-β1 (A-1A5), anti-β2 (MEM48), or anti-actin mAb (right).
Figure 2.
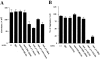
Anti-CD9 and -CD81 mAbs promote the fusion of blood monocytes. (A) Blood monocytes were induced to fuse into MGCs in culture medium containing 10 μg/ml Con A for 3 d in the absence or presence of 10 μg/ml of the indicated mAbs. Nuclei were then visualized using Wright stain. Bar, 250 μm. (B) Monocytes (2 × 105) were plated into the wells of a 96-well tissue culture plate and induced to fuse in the absence or presence of 10 μg/ml of the indicated mAbs. Fusion rates were determined by calculating the percentages of the number of nuclei within MGCs (three or more nuclei per cell) per total number of nuclei. (C) Monocytes were induced to fuse in the absence or presence of increasing concentrations of anti-CD9 (BU16), anti-CD81 (JS64) mAbs, or isotype-matched IgG, and then fusion rates were determined. (D) Monocytes were induced to fuse in the absence or presence of 10 μg/ml of the indicated mAbs (in the case of BU16 + JS64, 10 μg/ml of each was used). Fusion rates were then determined. Each bar and data point represent the mean ± SD.
Figure 2.
Anti-CD9 and -CD81 mAbs promote the fusion of blood monocytes. (A) Blood monocytes were induced to fuse into MGCs in culture medium containing 10 μg/ml Con A for 3 d in the absence or presence of 10 μg/ml of the indicated mAbs. Nuclei were then visualized using Wright stain. Bar, 250 μm. (B) Monocytes (2 × 105) were plated into the wells of a 96-well tissue culture plate and induced to fuse in the absence or presence of 10 μg/ml of the indicated mAbs. Fusion rates were determined by calculating the percentages of the number of nuclei within MGCs (three or more nuclei per cell) per total number of nuclei. (C) Monocytes were induced to fuse in the absence or presence of increasing concentrations of anti-CD9 (BU16), anti-CD81 (JS64) mAbs, or isotype-matched IgG, and then fusion rates were determined. (D) Monocytes were induced to fuse in the absence or presence of 10 μg/ml of the indicated mAbs (in the case of BU16 + JS64, 10 μg/ml of each was used). Fusion rates were then determined. Each bar and data point represent the mean ± SD.
Figure 2.
Anti-CD9 and -CD81 mAbs promote the fusion of blood monocytes. (A) Blood monocytes were induced to fuse into MGCs in culture medium containing 10 μg/ml Con A for 3 d in the absence or presence of 10 μg/ml of the indicated mAbs. Nuclei were then visualized using Wright stain. Bar, 250 μm. (B) Monocytes (2 × 105) were plated into the wells of a 96-well tissue culture plate and induced to fuse in the absence or presence of 10 μg/ml of the indicated mAbs. Fusion rates were determined by calculating the percentages of the number of nuclei within MGCs (three or more nuclei per cell) per total number of nuclei. (C) Monocytes were induced to fuse in the absence or presence of increasing concentrations of anti-CD9 (BU16), anti-CD81 (JS64) mAbs, or isotype-matched IgG, and then fusion rates were determined. (D) Monocytes were induced to fuse in the absence or presence of 10 μg/ml of the indicated mAbs (in the case of BU16 + JS64, 10 μg/ml of each was used). Fusion rates were then determined. Each bar and data point represent the mean ± SD.
Figure 3.
Anti-CD9 and -CD81 mAbs do not affect monocyte adhesion or aggregation. (A) Blood monocytes (2 × 105) were suspended in RPMI 1640 containing 10 μg/ml Con A, and were allowed to adhere to the wells of a 96-well culture plate for 12 h in the absence or presence of 10 μg/ml of the indicated mAbs. Nonadherent cells were removed, and the remaining adherent cells were evaluated using an MTT assay. (B) Monocytes (2 × 105) were cultured in RPMI 1640 containing 5 μg/ml Con A in the absence or presence of 10 μg/ml mAbs for 12 h on the wells of a 96-well nontissue culture-treated plate. The numbers of cell aggregates (>4 cells/aggregate) were determined under a light microscope. Each bar represents the mean ± SD.
Figure 4.
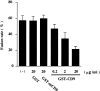
Soluble GST–CD9 large extracellular loop protein inhibits monocyte fusion. Blood monocytes were induced to fuse into MGCs in culture medium containing Con A for 3 d in the absence or presence of the indicated concentrations of GST, GST–mCD9, or GST–CD9. Fusion rates were then determined. A higher concentration (15 μg/ml) of Con A was used in order to show the dose-dependent, fusion-inhibitory effect of GST–CD9. Each bar represents the mean ± SD.
Figure 5.
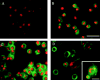
Modulation of the expression and distribution of CD9 in murine alveolar macrophages under fusogenic conditions. Murine alveolar macrophages were cultured in DME containing 5% human serum for 3 d under normal conditions (C) or fusogenic conditions containing 1α,25(OH)2D3 and culture supernatant from Con A–stimulated splenocytes (D). Macrophages were immunostained at d 0 (A and B) and at d 3 (C and D) with control mAb (A) or anti-CD9 mAb (B–D). Cells and nuclei were visualized with FITC-conjugated anti–rat immunoglobulin and propidium iodide. Typical CD9 distribution in an MGC is shown in the inset. Bar, 250 μm (100 μm for the inset). (E) Murine alveolar macrophages were cultured in the absence or presence of 1α,25(OH)2D3 and supernatant (SN) from Con A–stimulated splenocytes. After the indicated number of days, cells were lysed, and whole-cell lysates containing equal amounts of protein were separated by SDS-PAGE, transferred to a membrane, and probed with anti-CD9 mAb (KMC8).
Figure 5.
Modulation of the expression and distribution of CD9 in murine alveolar macrophages under fusogenic conditions. Murine alveolar macrophages were cultured in DME containing 5% human serum for 3 d under normal conditions (C) or fusogenic conditions containing 1α,25(OH)2D3 and culture supernatant from Con A–stimulated splenocytes (D). Macrophages were immunostained at d 0 (A and B) and at d 3 (C and D) with control mAb (A) or anti-CD9 mAb (B–D). Cells and nuclei were visualized with FITC-conjugated anti–rat immunoglobulin and propidium iodide. Typical CD9 distribution in an MGC is shown in the inset. Bar, 250 μm (100 μm for the inset). (E) Murine alveolar macrophages were cultured in the absence or presence of 1α,25(OH)2D3 and supernatant (SN) from Con A–stimulated splenocytes. After the indicated number of days, cells were lysed, and whole-cell lysates containing equal amounts of protein were separated by SDS-PAGE, transferred to a membrane, and probed with anti-CD9 mAb (KMC8).
Figure 6.
Anti-CD9 and -CD81 mAbs promote the fusion of murine alveolar macrophages. (A) Murine alveolar macrophages were induced to fuse into MGCs under fusogenic conditions containing 1α,25(OH)2D3 and culture supernatant from Con A–stimulated splenocytes for 3 d in the absence or presence of 10 μg/ml of the indicated mAbs. Nuclei were then visualized using Wright stain. Bar, 250 μm. (B) Murine alveolar macrophages (3 × 105) were plated into the wells of a 96-well culture plate and induced to fuse in the absence or presence of 10 μg/ml control IgG or various anti-CD9 and -CD81 mAbs (in the case of KMC8 + 2F7, 10 μg/ml of each was used). The numbers of MGCs per well were determined. Each bar represents the mean ± SD.
Figure 6.
Anti-CD9 and -CD81 mAbs promote the fusion of murine alveolar macrophages. (A) Murine alveolar macrophages were induced to fuse into MGCs under fusogenic conditions containing 1α,25(OH)2D3 and culture supernatant from Con A–stimulated splenocytes for 3 d in the absence or presence of 10 μg/ml of the indicated mAbs. Nuclei were then visualized using Wright stain. Bar, 250 μm. (B) Murine alveolar macrophages (3 × 105) were plated into the wells of a 96-well culture plate and induced to fuse in the absence or presence of 10 μg/ml control IgG or various anti-CD9 and -CD81 mAbs (in the case of KMC8 + 2F7, 10 μg/ml of each was used). The numbers of MGCs per well were determined. Each bar represents the mean ± SD.
Figure 7.
Enhanced cell fusion by CD9- and CD81-null murine alveolar macrophages and bone marrow cells after in vitro or in vivo stimulation. (A) Alveolar macrophages (3 × 105) from wild-type, CD9 (−/−), and CD81 (−/−) mice were plated into the wells of a 96-well culture plate and induced to fuse into MGCs by a 3-d incubation with 1α,25(OH)2D3 and splenocyte-conditioned medium. Nuclei were then visualized using Wright stain (left). The numbers of MGCs per well were determined (right). (B) 300 μg heat-killed P. acnes was administered intratracheally to wild-type, CD9 (−/−), and CD81 (−/−) mice. After 7 d, lung paraffin sections were stained with hematoxylin and eosin (left). Arrowheads indicate MGCs. In a separate experiment, alveolar macrophages were isolated by BAL from the lung, and the numbers of MGCs per lung were determined (right). Assays were done in triplicate for each animal tested. Two additional experiments gave similar results. (C) Bone marrow cells (2 × 106) from wild-type, CD9 (−/−), and CD81 (−/−) mice were plated into the wells of a 24-well culture plate and induced to fuse by a 7-d incubation with sRANKL and M-CSF. Cells were then stained for TRAP (left). The numbers of TRAP-positive multinucleated cells (MNCs) per well were determined (right). Bar, 100 μm (A–C). Each bar represents the mean ± SD.
Figure 7.
Enhanced cell fusion by CD9- and CD81-null murine alveolar macrophages and bone marrow cells after in vitro or in vivo stimulation. (A) Alveolar macrophages (3 × 105) from wild-type, CD9 (−/−), and CD81 (−/−) mice were plated into the wells of a 96-well culture plate and induced to fuse into MGCs by a 3-d incubation with 1α,25(OH)2D3 and splenocyte-conditioned medium. Nuclei were then visualized using Wright stain (left). The numbers of MGCs per well were determined (right). (B) 300 μg heat-killed P. acnes was administered intratracheally to wild-type, CD9 (−/−), and CD81 (−/−) mice. After 7 d, lung paraffin sections were stained with hematoxylin and eosin (left). Arrowheads indicate MGCs. In a separate experiment, alveolar macrophages were isolated by BAL from the lung, and the numbers of MGCs per lung were determined (right). Assays were done in triplicate for each animal tested. Two additional experiments gave similar results. (C) Bone marrow cells (2 × 106) from wild-type, CD9 (−/−), and CD81 (−/−) mice were plated into the wells of a 24-well culture plate and induced to fuse by a 7-d incubation with sRANKL and M-CSF. Cells were then stained for TRAP (left). The numbers of TRAP-positive multinucleated cells (MNCs) per well were determined (right). Bar, 100 μm (A–C). Each bar represents the mean ± SD.
Figure 8.
Histological sections of the lung and bone from wild-type and CD9/CD81 double-null mice. Lung sections from wild-type (A) and CD9 (−/−)/CD81 (−/−) mice (B and C) aged 8 wk were stained with hematoxylin and eosin. Bone sections of the proximal tibia from wild-type (D) and CD9 (−/−)/CD81 (−/−) mice (E) were stained for TRAP. Arrowheads indicate typical MGCs (B and C) and osteoclasts (D and E). Bars: 250 μm for A and B, 100 μm for C–E.
Similar articles
- RANKL-induced expression of tetraspanin CD9 in lipid raft membrane microdomain is essential for cell fusion during osteoclastogenesis.
Ishii M, Iwai K, Koike M, Ohshima S, Kudo-Tanaka E, Ishii T, Mima T, Katada Y, Miyatake K, Uchiyama Y, Saeki Y. Ishii M, et al. J Bone Miner Res. 2006 Jun;21(6):965-76. doi: 10.1359/jbmr.060308. J Bone Miner Res. 2006. PMID: 16753027 - Distinct roles for tetraspanins CD9, CD63 and CD81 in the formation of multinucleated giant cells.
Parthasarathy V, Martin F, Higginbottom A, Murray H, Moseley GW, Read RC, Mal G, Hulme R, Monk PN, Partridge LJ. Parthasarathy V, et al. Immunology. 2009 Jun;127(2):237-48. doi: 10.1111/j.1365-2567.2008.02945.x. Immunology. 2009. PMID: 19489128 Free PMC article. - Reduced fertility of female mice lacking CD81.
Rubinstein E, Ziyyat A, Prenant M, Wrobel E, Wolf JP, Levy S, Le Naour F, Boucheix C. Rubinstein E, et al. Dev Biol. 2006 Feb 15;290(2):351-8. doi: 10.1016/j.ydbio.2005.11.031. Epub 2005 Dec 27. Dev Biol. 2006. PMID: 16380109 - CD81 (TAPA-1): a molecule involved in signal transduction and cell adhesion in the immune system.
Levy S, Todd SC, Maecker HT. Levy S, et al. Annu Rev Immunol. 1998;16:89-109. doi: 10.1146/annurev.immunol.16.1.89. Annu Rev Immunol. 1998. PMID: 9597125 Review. - [A crucial role of tetraspanin, CD9 in fertilization].
Miyado K, Mekada E, Kobayashi K. Miyado K, et al. Tanpakushitsu Kakusan Koso. 2000 Jul;45(10):1728-34. Tanpakushitsu Kakusan Koso. 2000. PMID: 10897685 Review. Japanese. No abstract available.
Cited by
- A CD63 Homolog Specially Recruited to the Fungi-Contained Phagosomes Is Involved in the Cellular Immune Response of Oyster Crassostrea gigas.
Liu C, Yang C, Wang M, Jiang S, Yi Q, Wang W, Wang L, Song L. Liu C, et al. Front Immunol. 2020 Jul 22;11:1379. doi: 10.3389/fimmu.2020.01379. eCollection 2020. Front Immunol. 2020. PMID: 32793193 Free PMC article. - Role of DC-STAMP in cellular fusion of osteoclasts and macrophage giant cells.
Yagi M, Miyamoto T, Toyama Y, Suda T. Yagi M, et al. J Bone Miner Metab. 2006;24(5):355-8. doi: 10.1007/s00774-006-0697-9. J Bone Miner Metab. 2006. PMID: 16937266 Review. - Monocyte Subsets Have Distinct Patterns of Tetraspanin Expression and Different Capacities to Form Multinucleate Giant Cells.
Champion TC, Partridge LJ, Ong SM, Malleret B, Wong SC, Monk PN. Champion TC, et al. Front Immunol. 2018 Jun 8;9:1247. doi: 10.3389/fimmu.2018.01247. eCollection 2018. Front Immunol. 2018. PMID: 29937768 Free PMC article. - Towards using 3D cellular cultures to model the activation and diverse functions of macrophages.
Cutter S, Wright MD, Reynolds NP, Binger KJ. Cutter S, et al. Biochem Soc Trans. 2023 Feb 27;51(1):387-401. doi: 10.1042/BST20221008. Biochem Soc Trans. 2023. PMID: 36744644 Free PMC article. Review. - Macrophage cell lines use CD81 in cell growth regulation.
Mordica WJ, Woods KM, Clem RJ, Passarelli AL, Chapes SK. Mordica WJ, et al. In Vitro Cell Dev Biol Anim. 2009 May-Jun;45(5-6):213-25. doi: 10.1007/s11626-008-9167-0. Epub 2009 Jan 30. In Vitro Cell Dev Biol Anim. 2009. PMID: 19184252 Free PMC article.
References
- Abe, E., C. Miyaura, H. Tanaka, Y. Shiina, T. Kuribayashi, S. Suda, Y. Nishii, H.F. DeLuca, and T. Suda. 1983. 1 alpha,25-dihydroxyvitamin D3 promotes fusion of mouse alveolar macrophages both by a direct mechanism and by a spleen cell-mediated indirect mechanism. Proc. Natl. Acad. Sci. USA. 80:5583–5587. - PMC - PubMed
- Abe, E., Y. Ishimi, C.H. Jin, M.H. Hong, T. Sato, and T. Suda. 1991. Granulocyte-macrophage colony-stimulating factor is a major macrophage fusion factor present in conditioned medium of concanavalin A-stimulated spleen cell cultures. J. Immunol. 147:1810–1815. - PubMed
- Anderson, J.M. 2000. Multinucleated giant cells. Curr. Opin. Hematol. 7:40–47. - PubMed
- Berditchevski, F., G. Bazzoni, and M.E. Hemler. 1995. Specific association of CD63 with the VLA-3 and VLA-6 integrins. J. Biol. Chem. 270:17784–17790. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous