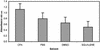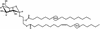A newly discovered cholesteryl galactoside from Borrelia burgdorferi - PubMed (original) (raw)
A newly discovered cholesteryl galactoside from Borrelia burgdorferi
Gil Ben-Menachem et al. Proc Natl Acad Sci U S A. 2003.
Abstract
Two major glycolipids, which comprise approximately 36% of the total lipid mass from Borrelia burgdorferi, the etiological agent of Lyme disease, were investigated. We determined the fatty acid type, sugar identity, anomeric configuration, and substituent type and position. The structures were identified as cholesteryl 6-O-acyl-beta-d-galactopyranoside (B. burgdorferi glycolipid 1, BbGL-I), and 1,2-di-O-acyl-3-O-alpha-d-galactopyranosyl-sn-glycerol (BbGL-II). The major fatty acids were palmitate and oleate. The structures were corroborated by gas-liquid chromatography MS, matrix-assisted laser desorption/ionization time-of-flight spectroscopy, fast atom bombardment MS, detailed NMR spectrometry, and metabolic labeling. This is a previously undescribed demonstration of a cholesteryl galactoside in bacteria. Lipopolysaccharide was not detected in B. burgdorferi. The two glycolipids have several properties suggesting they may function as lipopolysaccharide: both are main components of the bacterial membrane, surface exposed, and have a three-domain structure. BbGL-I elicited specific antibodies in mice and rabbits, and BbGL-II elicited antibodies that reacted with both glycolipids.
Figures
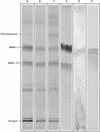
Fig. 1.
TLC analysis of lipid extracts from B. burgdorferi. Lipid extracts, visualized by iodine vapor, from strain B31 (A), strain N40 (B), strain BL303 (C), sprayed with anthrone reagent (strain B31) (D), immunostained with mouse anti BbGL-I (strain B31) (E), or lipid extract of Detoxy-Gel eluted material, exposed to iodine vapor (strain B31) (F).
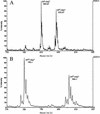
Fig. 2.
Part of the MALDI-TOF mass spectra of sodium adduct of BbGL-I and BbGL-II. Two major peaks of pseudomolecular ions [M+Na]+ at m/z 809.87 and 835.87 represent palmitic (MI) and oleic (MII) derivatives of cholesteryl galactoside (BbGL-I; A), and pseudomolecular ions [M+Na]+ at m/z 780.1 and 806.1 represent monogalactosyl diacyl glycerol (BbGL-II) containing dipalmitoyl (MIII) and palmitoyl-oleyl (MIV) substituents (B).
Fig. 3.
Antibody titers of sera from rabbits immunized with BbGL-I. Antibody titers were measured by an ELISA, as described in Experimental Procedures, and represent an average of four different rabbit sera, diluted 1:100, each tested in three independent assays. Open bars, IgG titers; gray bars, IgM titers; brackets, standard deviation.
Fig. 4.
Antibody titers of sera from mice immunized with BbGL-I. Mice (10 mice per group) were immunized as described in Experimental Procedures, with BbGL-I in Freund's adjuvant (CFA); PBS; DMSO, or squalene. Antibody titers were determined by an ELISA in sera diluted 1:100, and are represented as an average of three independent assays. Brackets, standard deviation.
Fig. 5.
Structure of BbGL-I. Cholesteryl 6-_O_-palmitoyl-β-
d
-galactopyranoside.
Fig. 6.
Structure of BbGL-II. 1-_O_-palmitoyl-2-_O_-oleyl-3-_O_-α-
d
-galactopyranosyl-_sn_-glycerol.
References
- Steere, A. C. (2001) N. Engl. J. Med. 345**,** 115-125. - PubMed
- Vinh, T. U., Shi, M. H., Adler, B. & Faine, S. (1989) J. Gen. Microbiol. 135**,** 2663-2673. - PubMed
- Cinco, M., Banfi, E., Balanzin, D., Godeas, C. & Panfili, E. (1991) FEMS Microbiol. Immunol. 3**,** 33-38. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases