Clinical aspects of a phase I trial of 5,6-dimethylxanthenone-4-acetic acid (DMXAA), a novel antivascular agent - PubMed (original) (raw)
Clinical Trial
. 2003 Jun 16;88(12):1844-50.
doi: 10.1038/sj.bjc.6600992.
P I Thompson, B C Baguley, B D Evans, V J Harvey, D J Porter, M R McCrystal, M Small, K Bellenger, L Gumbrell, G W Halbert, P Kestell; Phase I/II Trials Committee of Cancer Research UK
Affiliations
- PMID: 12799625
- PMCID: PMC2741109
- DOI: 10.1038/sj.bjc.6600992
Clinical Trial
Clinical aspects of a phase I trial of 5,6-dimethylxanthenone-4-acetic acid (DMXAA), a novel antivascular agent
M B Jameson et al. Br J Cancer. 2003.
Abstract
The antitumour action of 5,6-dimethylxanthenone-4-acetic acid (DMXAA) is mediated through tumour-selective antivascular effects and cytokine induction. This clinical phase I trial was conducted to examine its toxicity, maximum tolerated dose, pharmacokinetics (PK) and pharmacodynamics (PD). A secondary objective was to assess its antitumour efficacy. DMXAA was administered every 3 weeks as a 20-min i.v. infusion. Dose escalation initially followed a modified Fibonacci schema but was also guided by PK and toxicity. A total of 63 patients received 161 courses of DMXAA over 19 dose levels ranging from 6 to 4900 mg m(-2). DMXAA was well tolerated at lower doses and no drug-related myelosuppression was seen. Rapidly reversible dose-limiting toxicities were observed at 4900 mg m(-2), including confusion, tremor, slurred speech, visual disturbance, anxiety, urinary incontinence and possible left ventricular failure. Transient prolongation of the corrected cardiac QT interval was seen in 13 patients evaluated at doses of 2000 mg m(-2) and above. A patient with metastatic cervical carcinoma achieved an unconfirmed partial response at 1100 mg m(-2), progressing after eight courses. The results of PK and PD studies are reported separately. DMXAA has antitumour activity at well-tolerated doses.
Figures
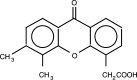
Figure 1
Structure of DMXAA.
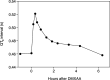
Figure 2
Change in corrected QT interval after DMXAA administration at 3700 mg m−2.
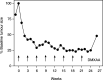
Figure 3
Tumour response in a patient with metastatic squamous carcinoma of cervix treated with DMXAA 1100 mg m−2. Tumour size was calculated as the sum of the products of bidimensional measurements of three clinically measurable metastatic neck nodes.
Similar articles
- 5,6-dimethylxanthenone-4-acetic acid (DMXAA): a new biological response modifier for cancer therapy.
Zhou S, Kestell P, Baguley BC, Paxton JW. Zhou S, et al. Invest New Drugs. 2002 Aug;20(3):281-95. doi: 10.1023/a:1016215015530. Invest New Drugs. 2002. PMID: 12201491 Review. - 5,6-dimethylxanthenone-4-acetic acid (DMXAA), a novel antivascular agent: phase I clinical and pharmacokinetic study.
Rustin GJ, Bradley C, Galbraith S, Stratford M, Loadman P, Waller S, Bellenger K, Gumbrell L, Folkes L, Halbert G; Phase I/II Trials Committee of Cancer Research UK. Rustin GJ, et al. Br J Cancer. 2003 Apr 22;88(8):1160-7. doi: 10.1038/sj.bjc.6600885. Br J Cancer. 2003. PMID: 12698178 Free PMC article. Clinical Trial. - Oral activity and pharmacokinetics of 5,6-dimethylxanthenone-4-acetic acid (DMXAA) in mice.
Zhao L, Kestell P, Ching LM, Baguley BC. Zhao L, et al. Cancer Chemother Pharmacol. 2002 Jan;49(1):20-6. doi: 10.1007/s00280-001-0377-3. Cancer Chemother Pharmacol. 2002. PMID: 11855749 - The antitumour activity of 5,6-dimethylxanthenone-4-acetic acid (DMXAA) in TNF receptor-1 knockout mice.
Zhao L, Ching LM, Kestell P, Baguley BC. Zhao L, et al. Br J Cancer. 2002 Aug 12;87(4):465-70. doi: 10.1038/sj.bjc.6600479. Br J Cancer. 2002. PMID: 12177785 Free PMC article. - Antivascular therapy of cancer: DMXAA.
Baguley BC. Baguley BC. Lancet Oncol. 2003 Mar;4(3):141-8. doi: 10.1016/s1470-2045(03)01018-0. Lancet Oncol. 2003. PMID: 12623359 Review.
Cited by
- Identification of new benzofuran derivatives as STING agonists with broad-spectrum antiviral activity.
Paulis A, Onali A, Vidalain PO, Lotteau V, Jaquemin C, Corona A, Distinto S, Delogu GL, Tramontano E. Paulis A, et al. Virus Res. 2024 Sep;347:199432. doi: 10.1016/j.virusres.2024.199432. Epub 2024 Jul 8. Virus Res. 2024. PMID: 38969014 Free PMC article. - Vascular disrupting agents in clinical development.
Hinnen P, Eskens FA. Hinnen P, et al. Br J Cancer. 2007 Apr 23;96(8):1159-65. doi: 10.1038/sj.bjc.6603694. Epub 2007 Mar 20. Br J Cancer. 2007. PMID: 17375046 Free PMC article. Review. - STING Agonists/Antagonists: Their Potential as Therapeutics and Future Developments.
Guerini D. Guerini D. Cells. 2022 Mar 29;11(7):1159. doi: 10.3390/cells11071159. Cells. 2022. PMID: 35406723 Free PMC article. Review. - The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by Tumor-Vascular Disrupting Agents.
Siemann DW. Siemann DW. Cancer Treat Rev. 2011 Feb;37(1):63-74. doi: 10.1016/j.ctrv.2010.05.001. Epub 2010 Jun 8. Cancer Treat Rev. 2011. PMID: 20570444 Free PMC article. Review. - Targeted disruption of tumor vasculature via polyphenol nanoparticles to improve brain cancer treatment.
Liu F, Peng B, Li M, Ma J, Deng G, Zhang S, Sheu WC, Zou P, Wu H, Liu J, Chen AT, Mohammed FS, Zhou J. Liu F, et al. Cell Rep Phys Sci. 2022 Jan 19;3(1):100691. doi: 10.1016/j.xcrp.2021.100691. Epub 2021 Dec 13. Cell Rep Phys Sci. 2022. PMID: 35199059 Free PMC article.
References
- Algire GH, Legallais FY, Park HD (1947) Vascular reactions of normal and malignant tissues in vivo. II. The vascular reaction of normal and neoplastic tissues of mice to a bacterial polysaccharide from Serratia marcescens (Bacillus prodigiosus) culture filtrates. J Natl Cancer Inst 8: 53–62
- Anderson H, Jap J, Price P (2000) Measurement of tumour and normal tissue (NT) perfusion by positron emission tomography (PET) in the evaluation of antivascular therapy: results in the phase I study of combretastatin A4 phosphate (CA4P). Proc Annu Meet Am Soc Clin Oncol 19: 179
- Aytemir K, Maarouf N, Gallagher MM, Yap YG, Waktare JE, Malik M (1999) Comparison of formulae for heart rate correction of QT interval in exercise electrocardiograms. Pacing Clin Electrophysiol 22: 1397–1401 - PubMed
- Baguley BC, Zhuang L, Kestell P (1997) Increased plasma serotonin following treatment with flavone-8-acetic acid, 5,6-dimethylxanthenone-4-acetic acid, vinblastine, and colchicine–relation to vascular effects. Oncol Res 9: 55–60 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources