COPI recruitment is modulated by a Rab1b-dependent mechanism - PubMed (original) (raw)
COPI recruitment is modulated by a Rab1b-dependent mechanism
Cecilia Alvarez et al. Mol Biol Cell. 2003 May.
Abstract
The small GTPase Rab1b is essential for endoplasmic reticulum (ER) to Golgi transport, but its exact function remains unclear. We have examined the effects of wild-type and three mutant forms of Rab1b in vivo. We show that the inactive form of Rab1b (the N121I mutant with impaired guanine nucleotide binding) blocks forward transport of cargo and induces Golgi disruption. The phenotype is analogous to that induced by brefeldin A (BFA): it causes resident Golgi proteins to relocate to the ER and induces redistribution of ER-Golgi intermediate compartment proteins to punctate structures. The COPII exit machinery seems to be functional in cells expressing the N121I mutant, but COPI is compromised, as shown by the release of beta-COP into the cytosol. Our results suggest that Rab1b function influences COPI recruitment. In support of this, we show that the disruptive effects of N121I can be reversed by expressing known mediators of COPI recruitment, the GTPase ARF1 and its guanine nucleotide exchange factor GBF1. Further evidence is provided by the finding that cells expressing the active form of Rab1b (the Q67L mutant with impaired GTPase activity) are resistant to BFA. Our data suggest a novel role for Rab1b in ARF1- and GBF1-mediated COPI recruitment pathway.
Figures
Figure 1.
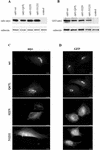
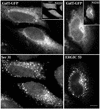
Expression and localization of wild-type and mutant Rab1b. HeLa cells were transfected with myc- or GFP-tagged wild-type Rab1b, the Q67L mutant, the S22N mutant, or the N121I mutant and analyzed 24 h later by Western blotting (A and B) or immunofluorescence (C and D). Cell lysates were obtained and processed by SDS-PAGE and immunoblotted with anti-myc and anti-calnexin antibodies (A) or with anti-GFP and anti-calnexin antibodies (B). Control lanes contain lysates from untransfected cells. Levels of expression of all constructs are similar. Cells were processed for immunofluorescence by use of anti-myc antibodies (C) or GFP fluorescence (D). Wild-type Rab1b and the Q67L mutant localize to the Golgi. The S22N mutant is detected in the cytosol and also in a reticulate pattern. The N121I mutant shows diffuse cytosol staining.
Figure 2.
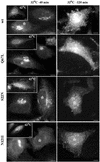
Rab1b mutants block cargo transport. HeLa cells were transfected with wild-type Rab1b, the Q67L mutant, the S22N mutant, or the N121I mutant. After 24 h, cells were infected with VSVtsO45 at 42°C. After 3 h, cells were either fixed (insets), incubated at 32°C for 40 min (32°C-40 min panels), or incubated at 32°C for 120 min (32°C-120 min panels) before fixation. Cells were analyzed by immunofluorescence with anti-myc antibodies to detect transfected cells (asterisks) and with anti-VSV-G antibodies. In cells expressing wild-type Rab1b or the Q67L mutant, VSV-G is transported to the Golgi at 40 min and to the cell surface at 120 min. In cells expressing the S22N mutant, VSV-G is transported out of the ER into punctate structures at 40 min and remains in such structures at 120 min. In cells expressing the N121I mutant, VSV-G is retained in the ER at 40 min and at 120 min. Bars, 10 μm.
Figure 3.
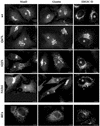
Rab1b mutants induce Golgi disruption. HeLa cells were transfected with the wild-type Rab1b, the Q67L mutant, the S22N mutant, or the N121I mutant. Cell were analyzed 24 h later by immunofluorescence with anti-myc antibodies to detect transfected cells (asterisks) and with anti-mannosidase II (anti-MannII), antigiantin, or anti-ERGIC53 antibodies (see respective panels). Wild-type Rab1b and the Q67L mutant do not affect the structure of the Golgi or ERGIC. The S22N mutant partially disrupts Golgi and induces the formation of punctate structures in the Golgi region. The N121I mutant causes complete Golgi disruption, with MannII and giantin redistributing to the ER and ERGIC53 relocating to peripheral punctate structures. The distribution of Golgi and ERGIC markers in cells expressing the N121I mutant is similar to that induced by a 30-min BFA (5 μg/ml) treatment (BFA panels). Bars, 10 μm.
Figure 4.
Rab1b mutants induce redistribution of its effectors GM130 and p115. HeLa cells were transfected with wild-type Rab1b, the Q67L mutant, the S22N mutant, or the N121I mutant and analyzed 24 h later by immunofluorescence with anti-myc antibodies to detect transfected cells (asterisks) and with either anti-GM130 or anti-p115 antibodies (see respective panels). In cells expressing wild-type Rab1b or the Q67L mutant, GM130 localizes to the Golgi, whereas p115 is found in the Golgi and in peripheral punctate structures. In cells expressing the S22N mutant, GM130 and p115 are partially localized to the Golgi but also show redistribution to peripheral punctate structures. In cells expressing the N121I mutant, both GM130 and p115 are completely redistributed to peripheral punctate structures. The redistribution of GM130 and p115 is similar to that induced by a 30-min BFA (5 μg/ml) treatment (BFA panels). Bars, 10 μm.
Figure 5.
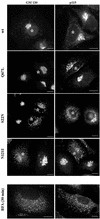
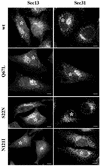
Rab1b mutants do not perturb COPII recruitment. HeLa cells were transfected with wild-type Rab1b, the Q67L mutant, the S22N mutant, or the N121I mutant and analyzed 24 h later by immunofluorescence with anti-myc antibodies to detect transfected cells (asterisks) and with either anti-Sec13 or anti-Sec31 antibodies (see respective panels). In cells expressing wild-type Rab1b or the Q67L mutant, COPII markers distribute in normal patterns. In cells expressing the S22N mutant or the N121I mutant, COPII markers present a relatively normal pattern but with fewer punctate structures in the Golgi region. Bars, 10 μm.
Figure 6.
The N121I mutant prevents sorting of a Golgi enzyme. HeLa cells were cotransfected with the N121I mutant and GFP-tagged Gal-T and analyzed 24 h later by immunofluorescence with anti-myc antibodies to detect transfected cells (insets) and with either anti-Sec31 or anti-ERGIC53 antibodies. Gal-T was detected by green fluorescence. Gal-T is localized to the ER in the same cells in which Sec31 or ERGIC53 present punctate patterns. Bars, 10 μm.
Figure 7.
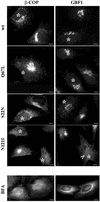
The N121I mutant induces β-COP dissociation and relocation of GBF1. HeLa cells were transfected with wild-type Rab1b, the Q67L mutant, the S22N mutant, or the N121I mutant (see respective panels) and analyzed 24 h later by immunofluorescence with anti-myc antibodies to detect transfected cells (asterisks) and with either anti-β-COP or anti-GBF1 antibodies. In cells expressing wild-type Rab1b or the Q67L mutant, β-COP and GBF1 distribute in normal patterns. In cells expressing the S22N mutant, β-COP and GBF1 present a more punctate peri-Golgi pattern. In cells expressing the N121I mutant, β-COP and GBF1 are not associated with a distinguishable compartment and appear diffuse. GBF1 is detected in the nuclear membrane (arrowhead), indicating ER localization. The redistribution of β-COP and GBF1 is similar to that induced by a 30-min BFA (5 μg/ml) treatment (BFA panels). Bars, 10 μm.
Figure 8.
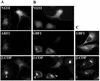
Expression of ARF1 or GBF1 rescues β-COP disassembly induced by the N121I mutant. HeLa cells were cotransfected with GFP-tagged N121I mutant and HA-tagged wild-type ARF1 (A) or with GFP-tagged N121I mutant and myc-tagged GBF1 (B). After 24 h, cells were analyzed by immunofluorescence with anti-β-COP antibodies and with either anti-HA or anti-myc antibodies. GFP-N121I was detected by green fluorescence. In cotransfected cells (arrowheads), β-COP is membrane associated. In cells transfected only with N121I (asterisks), β-COP is cytosolic. In C, cells were transfected only with myc-tagged GBF1. Overexpression of GBF1 causes partial Golgi fragmentation. Bars, 10 μm.
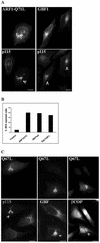
Figure 9.
Rab1-Q67L-transfected cells are BFA resistant. HeLa cells were transfected with HA-tagged ARF1-Q71L myc-tagged GBF1, or GFP-tagged Rab1b-Q67L. After 24 h, cells were treated with BFA (30 min; 5 μg/ml) and analyzed by immunofluorescence with either anti-HA or anti-myc antibodies to detect transfected cells and with anti-p115, anti-GBF1, or anti-β-COP antibodies to detect the Golgi. GFP-Rab1b-Q67L was detected by green fluorescence. Cells transfected with either ARF1-Q71L or GBF1 are BFA resistant, as shown by a Golgi pattern of p115 (arrowheads in A). The percentage of cells transfected with a control plasmid (encoding GFP), ARF1Q71L, GBF1, or Rab1-Q67L that are BFA resistant was quantified and is plotted as a bar graph (B). Bars represent the average of two independent experiments using p115 as a Golgi marker (50 transfected cells were counted in each experiment). Cells transfected with Rab1b-Q67L are BFA resistant, as shown by Golgi pattern of p115 and GBF1 and partial membrane association of β-COP (arrowheads in C). Bars, 10 μm.
Similar articles
- Rab1b interacts with GBF1 and modulates both ARF1 dynamics and COPI association.
Monetta P, Slavin I, Romero N, Alvarez C. Monetta P, et al. Mol Biol Cell. 2007 Jul;18(7):2400-10. doi: 10.1091/mbc.e06-11-1005. Epub 2007 Apr 11. Mol Biol Cell. 2007. PMID: 17429068 Free PMC article. - Rab1b-GBF1-ARF1 Secretory Pathway Axis Is Required for Birnavirus Replication.
Gimenez MC, Frontini-Lopez YR, Pocognoni CA, Roldán JS, García Samartino C, Uhart M, Colombo MI, Terebiznik MR, Delgui LR. Gimenez MC, et al. J Virol. 2022 Feb 23;96(4):e0200521. doi: 10.1128/JVI.02005-21. Epub 2021 Dec 8. J Virol. 2022. PMID: 34878889 Free PMC article. - Dissecting the role of the ARF guanine nucleotide exchange factor GBF1 in Golgi biogenesis and protein trafficking.
Szul T, Grabski R, Lyons S, Morohashi Y, Shestopal S, Lowe M, Sztul E. Szul T, et al. J Cell Sci. 2007 Nov 15;120(Pt 22):3929-40. doi: 10.1242/jcs.010769. Epub 2007 Oct 23. J Cell Sci. 2007. PMID: 17956946 - The COPI system: molecular mechanisms and function.
Beck R, Rawet M, Wieland FT, Cassel D. Beck R, et al. FEBS Lett. 2009 Sep 3;583(17):2701-9. doi: 10.1016/j.febslet.2009.07.032. Epub 2009 Jul 22. FEBS Lett. 2009. PMID: 19631211 Review. - ARF1 regulatory factors and COPI vesicle formation.
Spang A. Spang A. Curr Opin Cell Biol. 2002 Aug;14(4):423-7. doi: 10.1016/s0955-0674(02)00346-0. Curr Opin Cell Biol. 2002. PMID: 12383792 Review.
Cited by
- Rab1b interacts with GBF1 and modulates both ARF1 dynamics and COPI association.
Monetta P, Slavin I, Romero N, Alvarez C. Monetta P, et al. Mol Biol Cell. 2007 Jul;18(7):2400-10. doi: 10.1091/mbc.e06-11-1005. Epub 2007 Apr 11. Mol Biol Cell. 2007. PMID: 17429068 Free PMC article. - Altered ARA2 (RABA1a) expression in Arabidopsis reveals the involvement of a Rab/YPT family member in auxin-mediated responses.
Koh EJ, Kwon YR, Kim KI, Hong SW, Lee H. Koh EJ, et al. Plant Mol Biol. 2009 May;70(1-2):113-22. doi: 10.1007/s11103-009-9460-7. Epub 2009 Feb 6. Plant Mol Biol. 2009. PMID: 19199049 - The GTPase IFT27 is involved in both anterograde and retrograde intraflagellar transport.
Huet D, Blisnick T, Perrot S, Bastin P. Huet D, et al. Elife. 2014 Apr 24;3:e02419. doi: 10.7554/eLife.02419. Elife. 2014. PMID: 24843028 Free PMC article. - Spatiotemporal imaging of small GTPases activity in live cells.
Voss S, Krüger DM, Koch O, Wu YW. Voss S, et al. Proc Natl Acad Sci U S A. 2016 Dec 13;113(50):14348-14353. doi: 10.1073/pnas.1613999113. Epub 2016 Nov 29. Proc Natl Acad Sci U S A. 2016. PMID: 27911813 Free PMC article. - CREB3L1-mediated functional and structural adaptation of the secretory pathway in hormone-stimulated thyroid cells.
García IA, Torres Demichelis V, Viale DL, Di Giusto P, Ezhova Y, Polishchuk RS, Sampieri L, Martinez H, Sztul E, Alvarez C. García IA, et al. J Cell Sci. 2017 Dec 15;130(24):4155-4167. doi: 10.1242/jcs.211102. Epub 2017 Nov 1. J Cell Sci. 2017. PMID: 29093023 Free PMC article.
References
- Allan, B.B., Moyer, B.D., and Balch, W.E. (2000). Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science 289, 444–448. - PubMed
- Barbieri, M.A., Li, G., Mayorga, L.S., and Stahl, P.D. (1996). Characterization of Rab5:Q79L-stimulated endosome fusion. Arch. Biochem. Biophys. 326, 64–72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources