A novel boundary element may facilitate independent gene regulation in the Antennapedia complex of Drosophila - PubMed (original) (raw)
A novel boundary element may facilitate independent gene regulation in the Antennapedia complex of Drosophila
Vladimir E Belozerov et al. EMBO J. 2003.
Abstract
The intrinsic enhancer-promoter specificity and chromatin boundary/insulator function are two general mechanisms that govern enhancer trafficking in complex genetic loci. They have been shown to contribute to gene regulation in the homeotic gene complexes from fly to mouse. The regulatory region of the Scr gene in the Drosophila Antennapedia complex is interrupted by the neighboring ftz transcription unit, yet both genes are specifically activated by their respective enhancers from such juxtaposed positions. We identified a novel insulator, SF1, in the Scr-ftz intergenic region that restricts promoter selection by the ftz-distal enhancer in transgenic embryos. The enhancer-blocking activity of the full-length SF1, observed in both embryo and adult, is orientation- and enhancer-independent. The core region of the insulator, which contains a cluster of GAGA sites essential for its activity, is highly conserved among other Drosophila species. SF1 may be a member of a conserved family of chromatin boundaries/insulators in the HOM/Hox complexes and may facilitate the independent regulation of the neighboring Scr and ftz genes, by insulating the evolutionarily mobile ftz transcription unit.
Figures
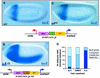
Fig. 1. Enhancer-blocking activity of SF1 in transgenic Drosophila embryos. (A) Schematic diagram of the ftz–Scr region. Arrows indicate the location and orientation of the Scr and ftz promoters. Open boxes represent exons and the thick lines represent introns and regulatory regions. Short vertical lines represent selected sites of several restriction enzymes, the names of which are indicated on the top right of the panel. Labeled boxes indicate selected enhancers and specialized DNA sequences: T.E., Scr Tethering Element (Calhoun et al., 2002); D, _ftz_-distal enhancer; A, ftz AE1 enhancer; T1, Scr enhancer for T1a; and PS2, Scr enhancer for C3p and T1a. The size and distance of the DNA elements are drawn to scale. (B–K) Reporter expression (white and eve/lacZ fusion gene) in blastoderm stage transgenic embryos visualized by whole-mount in situ hybridization (see Materials and methods). Embryos are shown anterior to the left and dorsal side up. Each test transgene is shown below the embryo image. (B and C) NLH embryos show a composite pattern consisting of comparable levels of NEE-directed ventral lateral expression and the anterior H1 stripe on the white (B) and lacZ (C) reporters. (D and E) NFH embryos show the reporter expression activated only by the proximal enhancers: NEE-directed ventrolateral stripes detected from white (D) and H1-specific expression from the lacZ reporter (E). (F and G) NFrevH embryos, which contain the SF1 element in reverse orientation, exhibit reporter expression by the proximal enhancers only: NEE from white (F), and H1 from lacZ (G). (H and I) PL3 embryos show a composite pattern consisting of PE-directed ventral expression and E3-directed mid-embryo stripe on the white (H) and lacZ (I) reporter genes. (J and K) PF3 embryos show that only the proximal enhancer can activate reporter expression: PE-directed ventral stain detected from white (J) and E3-specific expression from the lacZ reporter (K). (L) Quantitative assessment of the enhancer-blocking activity of each transgene. Thirty to 200 transgenic embryos from multiple lines were visually inspected for enhancer-blocking activity, which was categorized into weak, moderate or strong groups according to the level of reporter expression (see Materials and methods for details). The most frequently observed staining pattern is used in the figure.
Fig. 2. SF1 boundary activity in adult Drosophila. (A) The notum of a Canton-S adult female. Arrows indicate the macrochete bristles on the notum cuticle, both of which exhibit dark pigmentation. (B) The notum of a _yellow_1 adult female. Note the yellow-colored cuticle and bristles (arrows). (C) The notum of a transgenic adult female carrying the pYW-λ in a yellow mutant background (construct diagram shown on the top-right of the figure), showing the restored pigmentation in the bristles due to the activity of the B enhancer. (D) The notum of a transgenic adult carrying the pYW-SF1 transgene. The bristles are yellow, indicating the lack of yellow expression due to the blockage of the B enhancer by SF1. (E) The notum of a transgenic adult containing the pYW-Su(Hw) transgene. Similar yellow bristles are seen, indicating the Su(Hw)-mediated block of the B enhancer.
Fig. 3. Evolutionarily conserved core sequence of SF1 contains a cluster of GAGA sites. (A–J) Enhancer-blocking assay (only eve_–_lacZ reporter activity is shown) using the pNH vector was carried out (see Figure 1C) with SF1 sub-fragments inserted between the NEE and the H1 enhancers. (A–C) First round of enhancer-blocking tests using SF1/a (A), SF1/b (B) and SF1/c (C) fragments. The diagram above the embryos indicates the position and the size of the SF1/a, b and c fragments within the context of the full-length insulator (the orientation of the insulator is the same as in Figure 1A). Red boxes indicate GAGA sites. (D) Quantitative assessment of enhancer-blocking activity in the above transgenes with both eve–lacZ and the white reporter activity (see Figure 1 and methods for details). (E–G) Second round of enhancer-blocking tests using a single copy of SF1/b1 (E), SF1/b2 (F) and SF1/b3 (G) fragments. The diagram above the embryos indicates the position (orientation same as above) and the size of the SF1/b1, b2 and b3 fragments within the context of SF1/b. Note the terminal overlap between neighboring fragments. Red boxes indicate GAGA sites. (H–J) The enhancer-blocking activity of the trimerized fragments SF1/b1 (H), SF1/b2 (I) and SF1/b3 (J). (K) Quantitative assessment of the enhancer-blocking activity of the above transgenes using the eve–lacZ reporter activity (see Figure 1 and Materials and methods for details). (L) Sequence comparison of the SF1/b active fragment between four Drosophila species. Numbers represent the percent nucleotide identity between SF1/b1, SF1/b2 and SF1/b3 in the three species indicated and respective sequences in D.melanogaster. The conservation of the yellow gene coding region is given as an indication of evolutionary distance between the four fly species.
Fig. 4. The enhancer-blocking activity of the core SF1 depends on the GAGA factor. (A) LacZ expression in the wild-type embryo carrying the transgene shown diagrammatically below the figure. The reporter is expressed only in the domain of H1 enhancer, indicating a strong insulator activity of the SF1/b3 trimer. (B) The pattern of lacZ expression in the embryo carrying the knock-out transgene in which all nine GAGA sites present in the SF1/b3 trimer have been changed to the unrelated sequences. The reporter is expressed in both H1- and NEE-specific domains, demonstrating the lack of enhancer-blocking activity in the mutant insulator. (C) The homozygous males carrying the N(SF1/b3)3H transgene were mated with the hetero zygous _Trl_R85 females. The reporter expression in the F1 embryos was visualized by the whole-mount in situ hybridization. Compared to the wild type, a larger fraction of the mutant embryos exhibit NEE-induced lacZ expression, suggesting that the enhancer-blocking activity of SF1/b3 depends on the concentration of GAGA factor. (D) Quantitative assessment of the enhancer-blocking activity of the above transgenes using the eve–lacZ reporter activity (see Figure 1 and Materials and methods for details).
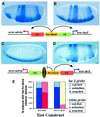
Fig. 5. Evidence for the potential role of SF1 in defining the range of the _ftz_-distal enhancer. (A and B) The expression of the white (A) and lacZ (B) reporters was visualized by whole-mount in situ hybridization of the embryos carrying the transgene shown below the figure. In the 2–4 h old embryos the _ftz_-distal enhancer activates the expression of the white and lacZ reporters at comparable levels. The other two enhancers present in this construct, E3 and PE, are also active, resulting in the composite expression pattern. (C and D) The insertion of SF1/b between PE and the _ftz_-distal enhancer redirects the enhancer trafficking in the test transgene. In the majority of embryos the white reporter (C) is activated by PE, whereas the lacZ reporter (D) is activated by the _ftz_-distal enhancer and E3. (E) Quantitative assessment of the enhancer-blocking activity in the above transgenes using the white and eve–lacZ reporter activity (see Figure 1 and Materials and methods for details).
Similar articles
- An Organizational Hub of Developmentally Regulated Chromatin Loops in the Drosophila Antennapedia Complex.
Li M, Ma Z, Liu JK, Roy S, Patel SK, Lane DC, Cai HN. Li M, et al. Mol Cell Biol. 2015 Dec;35(23):4018-29. doi: 10.1128/MCB.00663-15. Epub 2015 Sep 21. Mol Cell Biol. 2015. PMID: 26391952 Free PMC article. - Long-range enhancer-promoter interactions in the Scr-Antp interval of the Drosophila Antennapedia complex.
Calhoun VC, Levine M. Calhoun VC, et al. Proc Natl Acad Sci U S A. 2003 Aug 19;100(17):9878-83. doi: 10.1073/pnas.1233791100. Epub 2003 Aug 8. Proc Natl Acad Sci U S A. 2003. PMID: 12909726 Free PMC article. - Promoter-proximal tethering elements regulate enhancer-promoter specificity in the Drosophila Antennapedia complex.
Calhoun VC, Stathopoulos A, Levine M. Calhoun VC, et al. Proc Natl Acad Sci U S A. 2002 Jul 9;99(14):9243-7. doi: 10.1073/pnas.142291299. Epub 2002 Jul 1. Proc Natl Acad Sci U S A. 2002. PMID: 12093913 Free PMC article. - Unraveling cis-regulatory mechanisms at the abdominal-A and Abdominal-B genes in the Drosophila bithorax complex.
Akbari OS, Bousum A, Bae E, Drewell RA. Akbari OS, et al. Dev Biol. 2006 May 15;293(2):294-304. doi: 10.1016/j.ydbio.2006.02.015. Epub 2006 Mar 20. Dev Biol. 2006. PMID: 16545794 Review. - Chromatin insulators and long-distance interactions in Drosophila.
Kyrchanova O, Georgiev P. Kyrchanova O, et al. FEBS Lett. 2014 Jan 3;588(1):8-14. doi: 10.1016/j.febslet.2013.10.039. Epub 2013 Nov 5. FEBS Lett. 2014. PMID: 24211836 Review.
Cited by
- Study of the functional interaction between Mcp insulators from the Drosophila bithorax complex: effects of insulator pairing on enhancer-promoter communication.
Kyrchanova O, Toshchakov S, Parshikov A, Georgiev P. Kyrchanova O, et al. Mol Cell Biol. 2007 Apr;27(8):3035-43. doi: 10.1128/MCB.02203-06. Epub 2007 Feb 5. Mol Cell Biol. 2007. PMID: 17283051 Free PMC article. - Nuclear location of a chromatin insulator in Drosophila melanogaster.
Xu Q, Li M, Adams J, Cai HN. Xu Q, et al. J Cell Sci. 2004 Mar 1;117(Pt 7):1025-32. doi: 10.1242/jcs.00964. J Cell Sci. 2004. PMID: 14996934 Free PMC article. - A comprehensive map of insulator elements for the Drosophila genome.
Nègre N, Brown CD, Shah PK, Kheradpour P, Morrison CA, Henikoff JG, Feng X, Ahmad K, Russell S, White RA, Stein L, Henikoff S, Kellis M, White KP. Nègre N, et al. PLoS Genet. 2010 Jan 15;6(1):e1000814. doi: 10.1371/journal.pgen.1000814. PLoS Genet. 2010. PMID: 20084099 Free PMC article. - The enhancer-blocking activity of the Fab-7 boundary from the Drosophila bithorax complex requires GAGA-factor-binding sites.
Schweinsberg S, Hagstrom K, Gohl D, Schedl P, Kumar RP, Mishra R, Karch F. Schweinsberg S, et al. Genetics. 2004 Nov;168(3):1371-84. doi: 10.1534/genetics.104.029561. Genetics. 2004. PMID: 15579691 Free PMC article. - A variably occupied CTCF binding site in the ultrabithorax gene in the Drosophila bithorax complex.
Magbanua JP, Runneburger E, Russell S, White R. Magbanua JP, et al. Mol Cell Biol. 2015 Jan;35(1):318-30. doi: 10.1128/MCB.01061-14. Epub 2014 Nov 3. Mol Cell Biol. 2015. PMID: 25368383 Free PMC article.
References
- Akam M., Averof,M., Castelli-Gair,J., Dawes,R., Falciani,F. and Ferrier,D. (1994) The evolving role of Hox genes in arthropods. Dev. Suppl., 209–215. - PubMed
- Busturia A., Lloyd,A., Bejarano,F., Zavortink,M., Xin,H. and Sakonju,S. (2001) The MCP silencer of the Drosophila Abd-B gene requires both Pleiohomeotic and GAGA factor for the maintenance of repression. Development, 128, 2163–2173. - PubMed
- Cai H.N. and Shen,P. (2001) Effects of cis arrangement of chromatin insulators on enhancer-blocking activity. Science, 291, 493–495. - PubMed
- Cai H.N., Zhang,Z., Adams,J.R. and Shen,P. (2001) Genomic context modulates insulator activity through promoter competition. Development, 128, 4339–4347. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases