Tensile properties of Arabidopsis cell walls depend on both a xyloglucan cross-linked microfibrillar network and rhamnogalacturonan II-borate complexes - PubMed (original) (raw)
Tensile properties of Arabidopsis cell walls depend on both a xyloglucan cross-linked microfibrillar network and rhamnogalacturonan II-borate complexes
Peter Ryden et al. Plant Physiol. 2003 Jun.
Abstract
The mechanical properties of plant organs depend upon anatomical structure, cell-cell adhesion, cell turgidity, and the mechanical properties of their cell walls. By testing the mechanical responses of Arabidopsis mutants, it is possible to deduce the contribution that polymers of the cell wall make to organ strength. We developed a method to measure the tensile parameters of the expanded regions of turgid or plasmolyzed dark-grown Arabidopsis hypocotyls and applied it to the fucose biosynthesis mutant mur1, the xyloglucan glycosyltransferase mutants mur2 and mur3, and the katanin mutant bot1. Hypocotyls from plants grown in the presence of increasing concentrations of dichlorobenzonitrile, an inhibitor of cellulose synthesis, were considerably weakened, indicating the validity of our approach. In order of decreasing strength, the hypocotyls of mur2 > bot1 and mur1 > mur3 were each found to have reduced strength and a proportionate reduction in modulus compared with wild type. The tensile properties of the hypocotyls and of the inflorescence stems of mur1 were rescued by growth in the presence of high concentrations of borate, which is known to cross-link the pectic component rhamnogalacturonan II. From comparison of the mechanical responses of mur2 and mur3, we deduce that galactose-containing side chains of xyloglucan make a major contribution to overall wall strength, whereas xyloglucan fucosylation plays a comparatively minor role. We conclude that borate-complexed rhamnogalacturonan II and galactosylated xyloglucan contribute to the tensile strength of cell walls.
Figures
Figure 1.
Experiments to validate the tensile test method. A, Force displacement curve for the basal 3 mm of a 4-d-old dark-grown wild-type hypocotyl, expanded zone. 1, First movement, relieving tension; 2, second movement, the tensile test. B, Average strain distribution in: □, first (basal); ○, second; ▵, third; and •, fourth (apical) portions of two 4-d-old wild-type hypocotyls in which a strain was applied across the whole length of the hypocotyl. At the next increment, both had broken. C to E, Tensile properties of hypocotyls showing average strength and average modulus ± one
sd
(n = 20). C, Sections (2.5–2.8 mm) were tested from the lowest erect portion of 4-d-old wild-type hypocotyls (□) and from a position 3 mm above this (▪). Lower parts of 10-d-old dark-grown wild-type hypocotyls (○) were also tested; only the upper parts of 4-d-old hypocotyls were significantly different in strength from the lower portion at 4 d (P < 0.05). D, Four-day-old wild-type hypocotyls grown in the presence of 0 (□), 0.01 (▪), 0.1 (▴), or 0.25 (•) μ
m
DCB. E, Four-day-old bot1-1 hypocotyls (⋄) compared with wild type (□).
Figure 2.
Tensile properties and cellular phenotype of the hypocotyls of the mur mutants. A, Lower portions of 4-d-old wild-type hypocotyls and of the mur mutants (n = 20). □, Columbia (Col-0); ○, mur1-1; •, mur1-2; ×, mur2-1; ▵, mur3-1. Average tensile strength and modulus ± one
sd
are shown. B, Flaccid hypocotyls plasmolyzed in a 0.4
m
mannitol-supplemented medium for 6 h: ▵, mur3-1; and □, Col-0. C, Field emission scanning electron microscopy (FESEM) of fractured hypocotyls showing the anatomy of the endodermis (En) of wild type, mur1-1, mur2-1, and mur3-1 (bar = 10 μm). D, The fractured surface of a mur3-1 hypocotyl broken in a tensile test is shown between the arrows. A transverse cell wall is labeled with an asterisk (bar = 50 μm).
Figure 3.
Effects of borate on the primary wall properties of mur1-1 hypocotyls. Tensile properties of the lower portions of 4-d-old hypocotyls grown on media with various concentrations of borate: ○•, 0; □▪, 0.1 m
m
; □▪, 1.3 m
m
; ××, 2.6 m
m
; and ⋄♦, 5 m
m
boric acid. A, Black symbols, mur1-1; B, white symbols, Col-0.
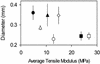
Figure 4.
An illustration of the difference in radial swelling among hypocotyls affected in wall composition. Average diameter of the basal 3-mm sections ± one
sd
and average moduli (n = 20) are shown for: wild type grown in: □, 0; ▪, 0.01; ▴, 0.1; and •, 0.25 μ
m
DCB; and the mutants: ○, mur1-1; ▵, mur3-1; and ⋄, bot1-1.
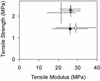
Figure 5.
Mechanical phenotypes of the pedicels of the mur mutants. Tensile properties of pedicels: □, Col-0; ○, mur1-1; •, mur1-2; ×, mur2-1; and ▵, mur3-1.
Similar articles
- Pectin may hinder the unfolding of xyloglucan chains during cell deformation: implications of the mechanical performance of Arabidopsis hypocotyls with pectin alterations.
Abasolo W, Eder M, Yamauchi K, Obel N, Reinecke A, Neumetzler L, Dunlop JW, Mouille G, Pauly M, Höfte H, Burgert I. Abasolo W, et al. Mol Plant. 2009 Sep;2(5):990-9. doi: 10.1093/mp/ssp065. Epub 2009 Sep 4. Mol Plant. 2009. PMID: 19825674 - The mur2 mutant of Arabidopsis thaliana lacks fucosylated xyloglucan because of a lesion in fucosyltransferase AtFUT1.
Vanzin GF, Madson M, Carpita NC, Raikhel NV, Keegstra K, Reiter WD. Vanzin GF, et al. Proc Natl Acad Sci U S A. 2002 Mar 5;99(5):3340-5. doi: 10.1073/pnas.052450699. Epub 2002 Feb 19. Proc Natl Acad Sci U S A. 2002. PMID: 11854459 Free PMC article. - The galactose residues of xyloglucan are essential to maintain mechanical strength of the primary cell walls in Arabidopsis during growth.
Peña MJ, Ryden P, Madson M, Smith AC, Carpita NC. Peña MJ, et al. Plant Physiol. 2004 Jan;134(1):443-51. doi: 10.1104/pp.103.027508. Plant Physiol. 2004. PMID: 14730072 Free PMC article. - Biosynthesis and properties of the plant cell wall.
Reiter WD. Reiter WD. Curr Opin Plant Biol. 2002 Dec;5(6):536-42. doi: 10.1016/s1369-5266(02)00306-0. Curr Opin Plant Biol. 2002. PMID: 12393017 Review. - Functions of xyloglucan in plant cells.
Hayashi T, Kaida R. Hayashi T, et al. Mol Plant. 2011 Jan;4(1):17-24. doi: 10.1093/mp/ssq063. Epub 2010 Oct 13. Mol Plant. 2011. PMID: 20943810 Review.
Cited by
- Multiscale characterization and micromechanical modeling of crop stem materials.
Gangwar T, Heuschele DJ, Annor G, Fok A, Smith KP, Schillinger D. Gangwar T, et al. Biomech Model Mechanobiol. 2021 Feb;20(1):69-91. doi: 10.1007/s10237-020-01369-6. Epub 2020 Aug 29. Biomech Model Mechanobiol. 2021. PMID: 32860537 Free PMC article. - KATANIN-dependent mechanical properties of the stigmatic cell wall mediate the pollen tube path in Arabidopsis.
Riglet L, Rozier F, Kodera C, Bovio S, Sechet J, Fobis-Loisy I, Gaude T. Riglet L, et al. Elife. 2020 Sep 1;9:e57282. doi: 10.7554/eLife.57282. Elife. 2020. PMID: 32867920 Free PMC article. - Aluminum Alters the Histology and Pectin Cell Wall Composition of Barley Roots.
Jaskowiak J, Kwasniewska J, Milewska-Hendel A, Kurczynska EU, Szurman-Zubrzycka M, Szarejko I. Jaskowiak J, et al. Int J Mol Sci. 2019 Jun 21;20(12):3039. doi: 10.3390/ijms20123039. Int J Mol Sci. 2019. PMID: 31234423 Free PMC article. - Boron deficiency in woody plants: various responses and tolerance mechanisms.
Wang N, Yang C, Pan Z, Liu Y, Peng S. Wang N, et al. Front Plant Sci. 2015 Oct 27;6:916. doi: 10.3389/fpls.2015.00916. eCollection 2015. Front Plant Sci. 2015. PMID: 26579163 Free PMC article. Review.
References
- Arioli T, Peng L, Betzner AS, Burn J, Wittke W, Herth W, Camilleri C, Höfte H, Plazinski J, et al. (1998) Molecular analysis of cellulose biosynthesis. Science 279: 717-720 - PubMed
- Bichet A, Desnos T, Turner S, Grandjean O, Höfte H (2001) BOTERO1 is required for normal orientation of cortical microtubules and anisotropic cell expansion in Arabidopsis. Plant J 25: 137-148 - PubMed
- Brown RP (1981) Handbook of Plastics Test Methods, Ed 2. George Godwin Ltd., London
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases