Rapid turnover of extracellular signal-regulated kinase 3 by the ubiquitin-proteasome pathway defines a novel paradigm of mitogen-activated protein kinase regulation during cellular differentiation - PubMed (original) (raw)
Rapid turnover of extracellular signal-regulated kinase 3 by the ubiquitin-proteasome pathway defines a novel paradigm of mitogen-activated protein kinase regulation during cellular differentiation
Philippe Coulombe et al. Mol Cell Biol. 2003 Jul.
Abstract
Mitogen-activated protein (MAP) kinases are stable enzymes that are mainly regulated by phosphorylation and subcellular targeting. Here we report that extracellular signal-regulated kinase 3 (ERK3), unlike other MAP kinases, is an unstable protein that is constitutively degraded in proliferating cells with a half-life of 30 min. The proteolysis of ERK3 is executed by the proteasome and requires ubiquitination of the protein. Contrary to other protein kinases, the catalytic activity of ERK3 is not responsible for its short half-life. Instead, analysis of ERK1/ERK3 chimeras revealed the presence of two destabilization regions (NDR1 and -2) in the N-terminal lobe of the ERK3 kinase domain that are both necessary and sufficient to target ERK3 and heterologous proteins for proteasomal degradation. To assess the physiological relevance of the rapid turnover of ERK3, we monitored the expression of the kinase in different cellular models of differentiation. We observed that ERK3 markedly accumulates during differentiation of PC12 and C2C12 cells into the neuronal and muscle lineage, respectively. The accumulation of ERK3 during myogenic differentiation is associated with the time-dependent stabilization of the protein. Terminal skeletal muscle differentiation is accompanied by cell cycle withdrawal. Interestingly, we found that expression of stabilized forms of ERK3 causes G(1) arrest in NIH 3T3 cells. We propose that ERK3 biological activity is regulated by its cellular abundance through the control of protein stability.
Figures
FIG. 1.
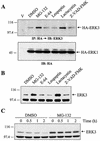
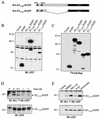
ERK3 is an unstable protein. (A) HEK 293 cells were transiently transfected with the indicated constructs, and cell lysates were analyzed by immunoblotting with anti-HA MAb. (B) Expression vectors for HA-ERK3 (E3) and HA-ERK1 (E1) were translated in vitro in the presence of [35S]methionine-cysteine and analyzed by fluorography. (C) HEK 293 cells were transfected with HA-ERK3 and HA-ERK1. After 48 h, the cells were treated with cycloheximide (100 μg/ml) for the indicated times. HA-ERK3 protein was immunoprecipitated with anti-HA MAb and detected by immunoblotting with a commercial anti-ERK3 antibody coupled to a biotin-streptavidin amplification system (upper panel). HA-ERK1 was analyzed as in panel A (lower panel). (D) Specificity of anti-ERK3 antibody. The polyclonal anti-ERK3 antibody E3-CTE4 was incubated in the absence (lane 1) or presence of the immunogen His6ERK3365-721GST (lane 2) or ERK3 N terminus (lane 3) prior to immunoblotting analysis of HEK 293 cell lysate. (E) HEK 293 (upper panel) and NIH 3T3 (lower panel) cells were treated with cycloheximide (100 μg/ml) for the indicated times. Endogenous ERK3 was detected by immunoblotting with a polyclonal antibody to the C terminus of ERK3 (E3-CTE4).
FIG. 2.
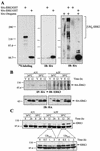
ERK3 is degraded by the proteasome. (A) HEK 293 cells were transfected with empty vector or with expression vectors for HA-ERK3 and HA-ERK1. After 48 h, the cells were treated for a period of 12 h with various protease inhibitors: MG-132 (25 μM), E-64 (25 μM), leupeptin (0.1 mM), lactacystin (20 μM), and Z-VAD-FMK (25 μM). Expression of HA-ERK3 protein was detected by immunoprecipitation with anti-HA MAb, followed by immunoblotting with anti-ERK3 antibody coupled to a biotin-streptavidin amplification system. Expression of ERK1 was analyzed by immunoblotting with anti-HA MAb. (B) HEK 293 cells were treated for 12 h with the indicated protease inhibitors. Endogenous ERK3 expression was detected by immunoblotting with E3-CTE4 antibody. (C) HEK 293 cells were treated with dimethyl sulfoxide (0.1%) or MG-132 (25 μM). After 30 min, cycloheximide was added to the medium for the times indicated. Expression of endogenous ERK3 was detected as in panel B.
FIG.3.
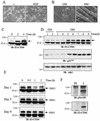
A functional ubiquitin-conjugating system is required for ERK3 proteolysis. (A) ERK3 is ubiquitinated in vivo. In the left panel is shown in vitro translation of expression vectors encoding HA-ERK3GST and HA-ERK1GST in the presence of [35S]methionine-cysteine. The products were detected by fluorography. In the central panel, HEK 293 cells were transfected with HA-ERK3GST and HA-ERK1GST expression constructs. After 48 h, GST-tagged proteins were precipitated with glutathione-agarose beads and analyzed by immunoblotting with anti-HA MAb. In the right panel, HEK 293 cells were cotransfected with GST-tagged expression vectors together with HA-ubiquitin. After 48 h, the cells were treated with MG-132 (25 μM) for an additional 12 h, and GST-tagged proteins were precipitated with glutathione-agarose beads. Immunoreactive ubiquitin conjugates were detected by immunoblotting with anti-HA MAb. (B) Parental BALB/c 3T3 (A31) and E1 mutant ts20 cells were infected with retroviruses encoding HA-ERK3 or HA-ERK1. At 3 days after selection in puromycin-containing medium, the infected cells were shifted or not at the restrictive temperature (39°C) for the times indicated. Expression of ectopic ERK3 and ERK1 proteins was detected as in Fig. 1E. (C) In the upper panel, A31 and ts20 cells were incubated at the permissive or restrictive temperature for the indicated times. Endogenous ERK3 expression was detected by immunoblotting with E3-CTE4 antibody. In the lower panel, ts20 cells were incubated at the permissive or restrictive temperature for 12 h. The cells were then treated with cycloheximide for the indicated times. Expression of endogenous ERK3 protein was monitored with E3-CTE4 antibody.
FIG. 4.
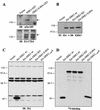
Role of Ser189 phosphorylation, kinase activity, and C-terminal extension on the stability of ERK3. (A) ERK3 is phosphorylated on Ser189. HEK 293 cells were transfected with GST fusion constructs of ERK3 wild-type or S189A mutant. GST-tagged proteins were purified from lysates by using glutathione-agarose beads and analyzed by immunoblotting with anti-phospho-ERK3(S189) (upper panel) or E3-CTD4 antibody (lower panel). (B) HEK 293 cells were transfected with the indicated expression constructs. Ectopically expressed ERK3 proteins were detected by immunoprecipitation with anti-HA MAb, followed by immunoblotting with anti-ERK3 antibody coupled to a biotin-streptavidin amplification system. (C) HEK 293 cells were transfected with the indicated expression constructs and cell lysates were analyzed by immunoblotting with anti-HA MAb. (D) The same expression constructs were translated in vitro in the presence of [35S]methionine-cysteine and analyzed by fluorography.
FIG. 5.
A signal located in the N terminus of ERK3 regulates its stability. (A) Schematic representation of the constructs used in these experiments. (B) In the upper panel, HEK 293 cells were transfected with the indicated expression constructs and cell lysates were analyzed by immunoblotting with anti-HA MAb. In the lower panel, in vitro translation of the same constructs was visualized by fluorography. (C) Synthesis of ERK1 and E31-73-E187-369 proteins. HEK 293 cells were transfected with the indicated constructs and pulse-labeled with [35S]methionine-cysteine for 5 min. The HA-tagged proteins were immunoprecipitated with anti-HA MAb and analyzed by fluorograply. (D) Turnover of ERK1 and E31-73-E187-369 proteins. Transfected HEK 293 cells were pulse-labeled for 2 h with [35S]methionine-cysteine and then chased for the indicated times in complete medium. The HA-tagged proteins were immunoprecipitated with anti-HA MAb and visualized with a PhosphorImager apparatus. (E) In the upper panel, HEK 293 cells were transfected with the indicated expression constructs, and lysates were analyzed by immunoblotting with anti-HA MAb. In the lower panel, in vitro translation of the same constructs was visualized by fluorography. (F) HEK 293 cells were transfected with an expression vector encoding HA-ERK11-201-ERK3201-721 chimera. After 48 h, the cells were treated with cycloheximide for the indicated times, and expression of the protein was detected by immunoblotting with anti-HA MAb.
FIG.6.
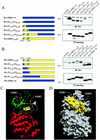
The N-terminal lobe of ERK3 kinase domain contains two regions that control protein stability. (A) Left panel, schematic representation of the constructs. In the upper right panel, HEK 293 cells were transfected with the indicated expression constructs, and cell lysates were analyzed by immunoblotting with anti-HA MAb. In the lower right panel is shown in vitro translation of the same constructs as visualized by fluorography. (B) Same as in panel A. (C) Ribbon diagram of ERK3 structure (amino acids 3 to 346) modeled from the X-ray structure of unphosphorylated ERK2. The N-terminal lobe is green, and the C-terminal lobe is red. NDR1 and NDR2 are yellow. (D) Surface representation of ERK3. NDR1 and NDR2 are yellow.
FIG. 7.
The N-terminal lobe of ERK3 kinase domain is sufficient to target proteins for rapid proteasomal degradation. (A) Schematic representation of the constructs used. (B) HEK 293 cells were transfected with the indicated expression constructs, and cell lysates were analyzed by immunoblotting with anti-GFP antibody. (C) The same expression constructs were translated in vitro in the presence of [35S]methionine-cysteine and analyzed by fluorography. (D) HEK 293 cells were transfected with expression vectors encoding HA-ERK31-112-EGFP and HA-ERK11-116-EGFP. After 48 h, the cells with treated with cycloheximide for the indicated times. The HA-ERK31-112-EGFP protein was detected by immunoprecipitation with anti-HA MAb, followed by immunoblotting with anti-GFP antibody coupled to a biotin-streptavidin amplification system (upper panel). HA-ERK11-116-EGFP was detected by immunoblotting with anti-GFP antibody (lower panel). (E) Transfected HEK 293 cells were treated for 12 h with the following protease inhibitors: MG-132 (25 μM), E-64 (25 μM), and lactacystin (20 μM). Expression of EGFP-tagged proteins was detected as in panel D.
FIG. 8.
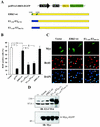
Induction of ERK3 expression during cellular differentiation. (A) PC12 cells were stimulated or not with NGF for 4 days. Phase-contrast micrographs show the outgrowth of neurites in NGF-treated cells. (B) C2C12 cells were grown in GM for 2 days or in DM for 7 days. Phase-contrast micrographs show the formation of myotubes in DM. (C) PC12 cells were treated with NGF for the indicated times. The expression of endogenous ERK3 was analyzed by immunoblotting with E3-CTE4 antibody. (D) C2C12 cells were incubated in GM or DM for the indicated times. The expression of endogenous ERK3, p21Cip1 and Cdk2 was analyzed by immunoblotting. (E) The half-life of ERK3 was evaluated by cycloheximide-chase experiments in proliferating (day 1) and differentiating (day 5 and 9) C2C12 myoblasts. (F) ERK3 is phosphorylated on Ser189 in differentiated muscle cells. Endogenous ERK3 was immunoprecipitated from differentiated C2C12 cells (day 9) with either preimmune serum (lane PI) or E3-CTE4 antibody (lane I). Phosphorylation of ERK3 was monitored by immunoblotting with anti-phospho-Ser189-specific antibody.
FIG.9.
Stable forms of ERK3 inhibit S-phase entry in NIH 3T3 fibroblasts. (A) Schematic representation of chimeric constructs used in these experiments. The NDR1 and NDR2 regions are highlighted. (B) NIH 3T3 cells were transfected with the indicated constructs. The cells were serum starved and then stimulated with 10% serum for 20 h in the presence of BrdU. The percentage of transfected cells (Myc-positive) that incorporated BrdU was evaluated by fluorescence microscopy. ns, not significantly different; ‡, P < 0.01 compared to vector; §, P < 0.01 compared to wild-type ERK3. (C) Representative example of the results shown in panel B. (D) NIH 3T3 cells were transfected or not with the indicated constructs. After 44 h, cell lysates were prepared and analyzed by immunoblotting with E3-CTE4 antibody (upper panel) and 9E10 MAb (lower panel).
FIG. 10.
Model of ERK3 regulation by proteasome-mediated degradation.
Similar articles
- Activation of MK5/PRAK by the atypical MAP kinase ERK3 defines a novel signal transduction pathway.
Seternes OM, Mikalsen T, Johansen B, Michaelsen E, Armstrong CG, Morrice NA, Turgeon B, Meloche S, Moens U, Keyse SM. Seternes OM, et al. EMBO J. 2004 Dec 8;23(24):4780-91. doi: 10.1038/sj.emboj.7600489. Epub 2004 Dec 2. EMBO J. 2004. PMID: 15577943 Free PMC article. - N-Terminal ubiquitination of extracellular signal-regulated kinase 3 and p21 directs their degradation by the proteasome.
Coulombe P, Rodier G, Bonneil E, Thibault P, Meloche S. Coulombe P, et al. Mol Cell Biol. 2004 Jul;24(14):6140-50. doi: 10.1128/MCB.24.14.6140-6150.2004. Mol Cell Biol. 2004. PMID: 15226418 Free PMC article. - Deubiquitinating Enzyme USP20 Regulates Extracellular Signal-Regulated Kinase 3 Stability and Biological Activity.
Mathien S, Déléris P, Soulez M, Voisin L, Meloche S. Mathien S, et al. Mol Cell Biol. 2017 Apr 14;37(9):e00432-16. doi: 10.1128/MCB.00432-16. Print 2017 May 1. Mol Cell Biol. 2017. PMID: 28167606 Free PMC article. - The extracellular signal-regulated kinase 3 (mitogen-activated protein kinase 6 [MAPK6])-MAPK-activated protein kinase 5 signaling complex regulates septin function and dendrite morphology.
Brand F, Schumacher S, Kant S, Menon MB, Simon R, Turgeon B, Britsch S, Meloche S, Gaestel M, Kotlyarov A. Brand F, et al. Mol Cell Biol. 2012 Jul;32(13):2467-78. doi: 10.1128/MCB.06633-11. Epub 2012 Apr 16. Mol Cell Biol. 2012. PMID: 22508986 Free PMC article. - Role of the Atypical MAPK ERK3 in Cancer Growth and Progression.
Elkhadragy L, Myers A, Long W. Elkhadragy L, et al. Cancers (Basel). 2024 Mar 31;16(7):1381. doi: 10.3390/cancers16071381. Cancers (Basel). 2024. PMID: 38611058 Free PMC article. Review.
Cited by
- Emerging roles of immunoproteasomes beyond MHC class I antigen processing.
Ebstein F, Kloetzel PM, Krüger E, Seifert U. Ebstein F, et al. Cell Mol Life Sci. 2012 Aug;69(15):2543-58. doi: 10.1007/s00018-012-0938-0. Epub 2012 Mar 2. Cell Mol Life Sci. 2012. PMID: 22382925 Free PMC article. Review. - The ubiquitin-proteasome system and signal transduction pathways regulating Epithelial Mesenchymal transition of cancer.
Voutsadakis IA. Voutsadakis IA. J Biomed Sci. 2012 Jul 24;19(1):67. doi: 10.1186/1423-0127-19-67. J Biomed Sci. 2012. PMID: 22827778 Free PMC article. Review. - Activation of MK5/PRAK by the atypical MAP kinase ERK3 defines a novel signal transduction pathway.
Seternes OM, Mikalsen T, Johansen B, Michaelsen E, Armstrong CG, Morrice NA, Turgeon B, Meloche S, Moens U, Keyse SM. Seternes OM, et al. EMBO J. 2004 Dec 8;23(24):4780-91. doi: 10.1038/sj.emboj.7600489. Epub 2004 Dec 2. EMBO J. 2004. PMID: 15577943 Free PMC article. - ERK3/MAPK6 controls IL-8 production and chemotaxis.
Bogucka K, Pompaiah M, Marini F, Binder H, Harms G, Kaulich M, Klein M, Michel C, Radsak MP, Rosigkeit S, Grimminger P, Schild H, Rajalingam K. Bogucka K, et al. Elife. 2020 Apr 21;9:e52511. doi: 10.7554/eLife.52511. Elife. 2020. PMID: 32314963 Free PMC article. - ERK3 signals through SRC-3 coactivator to promote human lung cancer cell invasion.
Long W, Foulds CE, Qin J, Liu J, Ding C, Lonard DM, Solis LM, Wistuba II, Qin J, Tsai SY, Tsai MJ, O'Malley BW. Long W, et al. J Clin Invest. 2012 May;122(5):1869-80. doi: 10.1172/JCI61492. Epub 2012 Apr 16. J Clin Invest. 2012. PMID: 22505454 Free PMC article.
References
- Blau, H. M., G. K. Pavlath, E. C. Hardeman, C. P. Chiu, L. Silberstein, S. G. Webster, S. C. Miller, and C. Webster. 1985. Plasticity of the differentiated state. Science 230:758-766. - PubMed
- Boulton, T. G., S. H. Nye, D. J. Robbins, N. Y. Ip, E. Radziejewska, S. D. Morgenbesser, R. A. DePinho, N. Panayotatos, M. H. Cobb, and G. D. Yancopoulos. 1991. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell 65:663-675. - PubMed
- Chang, L., and M. Karin. 2001. Mammalian MAP kinase signalling cascades. Nature 410:37-40. - PubMed
- Cheng, M., T. G. Boulton, and M. H. Cobb. 1996. ERK3 is a constitutively nuclear protein kinase. J. Biol. Chem. 271:8951-8958. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous