Bruton's tyrosine kinase is required for lipopolysaccharide-induced tumor necrosis factor alpha production - PubMed (original) (raw)
Bruton's tyrosine kinase is required for lipopolysaccharide-induced tumor necrosis factor alpha production
Nicole J Horwood et al. J Exp Med. 2003.
Abstract
Lipopolysaccharide (LPS), a product of Gram-negative bacteria, is potent mediator of tumor necrosis factor (TNF)alpha production by myeloid/macrophage cells. Inhibitors capable of blocking the signaling events that result in TNF alpha production could provide useful therapeutics for treating septic shock and other inflammatory diseases. Broad spectrum tyrosine inhibitors are known to inhibit TNF alpha production, however, no particular family of tyrosine kinases has been shown to be essential for this process. Here we show that the Bruton's tyrosine kinase (Btk)-deficient mononuclear cells from X-linked agammaglobulinemia patients have impaired LPS-induced TNF alpha production and that LPS rapidly induces Btk kinase activity in normal monocytes. In addition, adenoviral overexpression of Btk in normal human monocytes enhanced TNF alpha production. We examined the role of Btk in TNF alpha production using luciferase reporter adenoviral constructs and have established that overexpression of Btk results in the stabilization of TNF alpha mRNA via the 3' untranslated region. Stimulation with LPS also induced the activation of related tyrosine kinase, Tec, suggesting that the Tec family kinases are important components for LPS-induced TNF alpha production. This study provides the first clear evidence that tyrosine kinases of the Tec family, in particular Btk, are key elements of LPS-induced TNF alpha production and consequently may provide valuable therapeutic targets for intervention in inflammatory conditions.
Figures
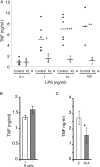
Figure 1.
LPS-induced TNFα expression in PBMCs/monocytes from XLA and normal donors. (A) PBMCs from XLA and normal male donors (age range 17–38 yr). (B) Total PBMCs and PBMCs depleted of B cells from normal donors. (C) Monocytes prepared by adherence from normal and XLA PBMCs. Cells were stimulated with 10 ng/ml LPS (unless otherwise stated) for 18 h and supernatants were assayed for TNFα production by ELISA. In A, each point represents a donor, B is representative of three separate experiments, and C shows the combined results of five donors of each type (error bars ± SD). Results from Student's t test P values: *, P < 0.05; **, P < 0.01; ***, P < 0.001. TNFα production from unactivated cells was undetectable.
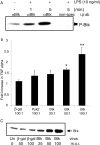
Figure 2.
Btk is activated by LPS stimulation and modulates TNFα production. (A) Primary monocytes were stimulated with LPS for the indicated intervals. Cell lysates were immunoprecipitated and immune complex kinase assays were performed. Autophosphorylated Btk proteins were detected by autoradiography. This result is representative of three separate experiments. (B) Monocytes were infected with adenoviruses encoding either wild-type Btk (Btk WT), β-galactosidase (β-Gal), or an unrelated tyrosine kinase, Pyk2. Cells were stimulated with LPS for 18 h and the supernatants were assayed for TNFα expression. Data is presented as fold activation compared with uninfected LPS stimulated control cells (±SD). *, P < 0.05; **, P < 0.01 compared with LPS-activated uninfected controls. (C) Cell lysates from the infected cells were examined for Btk expression by immuno-Western blotting. Data is representative of at least four different donors.
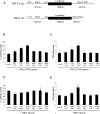
Figure 3.
LPS-induced IκBα degradation in XLA and normal PBMCs. (A) PBMCs stimulated with 10 ng/ml LPS for 20 min were lysed and sequentially immunoblotted for the indicated proteins. This result is representative of three different experiments. (B and C) M-CSF monocytes were simultaneously infected with NF-κB luciferase reporter construct (m.o.i. of 100) alone, or coinfected with either AdBtk or Ad0 at various m.o.i. 24 h after adenoviral infection, cells were treated with LPS for 18 h before assay of (B) luciferase activity and (C) TNFα production. Data are means of triplicate cultures ± SD and are expressed as a percentage of the control level. This graph is representative of five experiments performed using different donors.
Figure 3.
LPS-induced IκBα degradation in XLA and normal PBMCs. (A) PBMCs stimulated with 10 ng/ml LPS for 20 min were lysed and sequentially immunoblotted for the indicated proteins. This result is representative of three different experiments. (B and C) M-CSF monocytes were simultaneously infected with NF-κB luciferase reporter construct (m.o.i. of 100) alone, or coinfected with either AdBtk or Ad0 at various m.o.i. 24 h after adenoviral infection, cells were treated with LPS for 18 h before assay of (B) luciferase activity and (C) TNFα production. Data are means of triplicate cultures ± SD and are expressed as a percentage of the control level. This graph is representative of five experiments performed using different donors.
Figure 4.
Btk overexpression enhances TNFα via the 3′ UTR. (A) Schematic representation of the human TNF 5′ promoter-luciferase-3′ UTR and TNF 5′ promoter-luciferase adenoviral constructs. Activity of the human TNF 5′ 3′ UTR (B and C) or TNF 5′ promoter (D and E) in stimulated human macrophages was tested by simultaneously infecting primary human macrophages with reporter virus at an m.o.i. of 40 and either Ad0 or AdBtk at various m.o.i. 24 h after adenoviral infection, macrophages were treated with LPS for 18 h before assay of luciferase activity (B and D) and TNFα production (C and E). Data are means of triplicate cultures ± SD and are expressed as a percentage of the control level. Each graph is representative of five experiments performed using different donors.
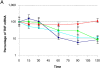
Figure 5.
Btk overexpression stabilizes TNFα mRNA. (A) Cells were either uninfected or infected with Ad0, Ad PykM, or AdBtk (m.o.i. of 100) and activated with LPS for 4 h. 5 μg/ml actinomycin D was added to stop any further mRNA synthesis and the cells were incubated for an additional 0, 15, 30, 60, 90, or 120 min, after which time they were harvested in RNA lysis buffer and the supernatants were reserved for TNFα ELISA. Total mRNA was prepared and Taqman RT-PCR was used to access the quantity of TNFα. The results were normalized to 100% at the 0-min time point. The lines are representative of Control (♦), Ad0 (▪), Btk (▴), and PykM (x). Data are means of triplicate reactions ± SD expressed as a percentage of the control and are representative of three experiments performed using different donors. (B) PBMCs from XLA and normal male donors or (C) M-CSF monocytes infected with AdBtk or AdPyk2, were stimulated with LPS for 20 min, lysed, and sequentially immunoblotted for the indicated proteins. These results are representative of three different experiments.
Figure 5.
Btk overexpression stabilizes TNFα mRNA. (A) Cells were either uninfected or infected with Ad0, Ad PykM, or AdBtk (m.o.i. of 100) and activated with LPS for 4 h. 5 μg/ml actinomycin D was added to stop any further mRNA synthesis and the cells were incubated for an additional 0, 15, 30, 60, 90, or 120 min, after which time they were harvested in RNA lysis buffer and the supernatants were reserved for TNFα ELISA. Total mRNA was prepared and Taqman RT-PCR was used to access the quantity of TNFα. The results were normalized to 100% at the 0-min time point. The lines are representative of Control (♦), Ad0 (▪), Btk (▴), and PykM (x). Data are means of triplicate reactions ± SD expressed as a percentage of the control and are representative of three experiments performed using different donors. (B) PBMCs from XLA and normal male donors or (C) M-CSF monocytes infected with AdBtk or AdPyk2, were stimulated with LPS for 20 min, lysed, and sequentially immunoblotted for the indicated proteins. These results are representative of three different experiments.
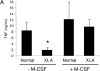
Figure 6.
The effect of M-CSF treatment on LPS-stimulated TNFα production and Tec expression. (A) Adherent monocytes from XLA and normal donors were either stimulated with LPS for 2 h, or incubated with M-CSF for 48 h before LPS stimulation for 2 h. Supernatants were assayed for TNFα production. (B) Adherent monocytes from normal (lanes 1 and 2) and XLA donors (lanes 3 and 4) were either lysed immediately, or after M-CSF treatment for 48 h. Western blot analysis was performed using either α-TecSH3, α-Btk, or α-actin antibody. (C) Elutriated monocytes from normal donors were stimulated with LPS for the indicated time periods. Tec was immunoprecipitated and in vitro autokinase assay was performed. Each study is representative of at least three separate experiments.
Figure 6.
The effect of M-CSF treatment on LPS-stimulated TNFα production and Tec expression. (A) Adherent monocytes from XLA and normal donors were either stimulated with LPS for 2 h, or incubated with M-CSF for 48 h before LPS stimulation for 2 h. Supernatants were assayed for TNFα production. (B) Adherent monocytes from normal (lanes 1 and 2) and XLA donors (lanes 3 and 4) were either lysed immediately, or after M-CSF treatment for 48 h. Western blot analysis was performed using either α-TecSH3, α-Btk, or α-actin antibody. (C) Elutriated monocytes from normal donors were stimulated with LPS for the indicated time periods. Tec was immunoprecipitated and in vitro autokinase assay was performed. Each study is representative of at least three separate experiments.
Similar articles
- Bruton's tyrosine kinase is not essential for LPS-induced activation of human monocytes.
Pérez de Diego R, López-Granados E, Pozo M, Rodríguez C, Sabina P, Ferreira A, Fontan G, García-Rodríguez MC, Alemany S. Pérez de Diego R, et al. J Allergy Clin Immunol. 2006 Jun;117(6):1462-9. doi: 10.1016/j.jaci.2006.01.037. Epub 2006 Mar 31. J Allergy Clin Immunol. 2006. PMID: 16751014 - LPS-Induced NF-kappaB activation and TNF-alpha release in human monocytes are protein tyrosine kinase dependent and protein kinase C independent.
Shames BD, Selzman CH, Pulido EJ, Meng X, Meldrum DR, McIntyre RC Jr, Harken AH, Banerjee A. Shames BD, et al. J Surg Res. 1999 May 1;83(1):69-74. doi: 10.1006/jsre.1998.5564. J Surg Res. 1999. PMID: 10210645 - Bruton's tyrosine kinase is required for TLR2 and TLR4-induced TNF, but not IL-6, production.
Horwood NJ, Page TH, McDaid JP, Palmer CD, Campbell J, Mahon T, Brennan FM, Webster D, Foxwell BM. Horwood NJ, et al. J Immunol. 2006 Mar 15;176(6):3635-41. doi: 10.4049/jimmunol.176.6.3635. J Immunol. 2006. PMID: 16517732 - Bruton's tyrosine kinase (Btk): function, regulation, and transformation with special emphasis on the PH domain.
Mohamed AJ, Yu L, Bäckesjö CM, Vargas L, Faryal R, Aints A, Christensson B, Berglöf A, Vihinen M, Nore BF, Smith CI. Mohamed AJ, et al. Immunol Rev. 2009 Mar;228(1):58-73. doi: 10.1111/j.1600-065X.2008.00741.x. Immunol Rev. 2009. PMID: 19290921 Review. - Bruton's tyrosine kinase: cell biology, sequence conservation, mutation spectrum, siRNA modifications, and expression profiling.
Lindvall JM, Blomberg KE, Väliaho J, Vargas L, Heinonen JE, Berglöf A, Mohamed AJ, Nore BF, Vihinen M, Smith CI. Lindvall JM, et al. Immunol Rev. 2005 Feb;203:200-15. doi: 10.1111/j.0105-2896.2005.00225.x. Immunol Rev. 2005. PMID: 15661031 Review.
Cited by
- MyD88 is involved in myeloid as well as lymphoid hematopoiesis independent of the presence of a pathogen.
Fiedler K, Kokai E, Bresch S, Brunner C. Fiedler K, et al. Am J Blood Res. 2013 May 5;3(2):124-40. Print 2013. Am J Blood Res. 2013. PMID: 23675564 Free PMC article. - Increased pro-inflammatory cytokine production after lipopolysaccharide stimulation in patients with X-linked agammaglobulinemia.
González-Serrano ME, Estrada-García I, Mogica-Martínez D, González-Garay A, López-Herrera G, Berrón-Ruiz L, Espinosa-Padilla SE, Yamazaki-Nakashimada MA, Vargas-Hernández A, Santos-Argumedo L, Estrada-Parra SA, Espinosa-Rosales FJ. González-Serrano ME, et al. J Clin Immunol. 2012 Oct;32(5):967-74. doi: 10.1007/s10875-012-9706-z. Epub 2012 Jun 5. J Clin Immunol. 2012. PMID: 22665224 - Tec kinases regulate actin assembly and cytokine expression in LPS-stimulated human neutrophils via JNK activation.
Zemans RL, Arndt PG. Zemans RL, et al. Cell Immunol. 2009;258(1):90-7. doi: 10.1016/j.cellimm.2009.03.017. Epub 2009 Apr 23. Cell Immunol. 2009. PMID: 19393603 Free PMC article. - The Bruton tyrosine kinase inhibitor PCI-32765 ameliorates autoimmune arthritis by inhibition of multiple effector cells.
Chang BY, Huang MM, Francesco M, Chen J, Sokolove J, Magadala P, Robinson WH, Buggy JJ. Chang BY, et al. Arthritis Res Ther. 2011 Jul 13;13(4):R115. doi: 10.1186/ar3400. Arthritis Res Ther. 2011. PMID: 21752263 Free PMC article. - Pyoderma Gangrenosum in a Patient with Bruton's X-linked Agammaglobulinemia: Shared Pathogenesis of Altered Tumor Necrosis Factor Alpha?
Schwartzfarb EM, Weir D, Conlan WA, Romanelli P, Kirsner RS. Schwartzfarb EM, et al. J Clin Aesthet Dermatol. 2008 May;1(1):26-9. J Clin Aesthet Dermatol. 2008. PMID: 21103306 Free PMC article.
References
- Feldmann, M., F.M. Brennan, and R.N. Maini. 1996. Rheumatoid arthritis. Cell. 85:307–310. - PubMed
- Beutler, B., and A. Cerami. 1988. Tumor necrosis, cachexia, shock, and inflammation: a common mediator. Annu. Rev. Biochem. 57:505–518. - PubMed
- Bondeson, J., B. Foxwell, F. Brennan, and M. Feldmann. 1999. Defining therapeutic targets by using adenovirus: blocking NF-kappaB inhibits both inflammatory and destructive mechanisms in rheumatoid synovium but spares anti-inflammatory mediators. Proc. Natl. Acad. Sci. USA. 96:5668–5673. - PMC - PubMed
- Foxwell, B., K. Browne, J. Bondeson, C. Clarke, R. de Martin, F. Brennan, and M. Feldmann. 1998. Efficient adenoviral infection with IkappaB alpha reveals that macrophage tumor necrosis factor alpha production in rheumatoid arthritis is NF-kappaB dependent. Proc. Natl. Acad. Sci. USA. 95:8211–8215. - PMC - PubMed
- Lee, J.C., J.T. Laydon, P.C. McDonnell, T.F. Gallagher, S. Kumar, D. Green, D. McNulty, M.J. Blumenthal, J.R. Heys, S.W. Landvatter, et al. 1994. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 372:739–746. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources