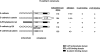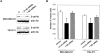Adhesion-independent mechanism for suppression of tumor cell invasion by E-cadherin - PubMed (original) (raw)
Adhesion-independent mechanism for suppression of tumor cell invasion by E-cadherin
Alice S T Wong et al. J Cell Biol. 2003.
Abstract
Loss of E-cadherin expression or function in tumors leads to a more invasive phenotype. In this study, we investigated whether the invasion suppressor activity of E-cadherin is mediated directly by tighter physical cell adhesion, indirectly by sequestering beta-catenin and thus antagonizing beta-catenin/T cell factor (TCF) signaling, or by other signaling pathways. To distinguish mechanisms, we expressed wild-type E-cadherin and various E-cadherin mutants in invasive E-cadherin-negative human breast (MDA-MB-231) and prostate (TSU-Pr1) epithelial carcinoma cell lines using a tetracycline-inducible system. Our data confirm that E-cadherin inhibits human mammary and prostate tumor cell invasion. We find that adhesion is neither necessary nor sufficient for suppressing cancer invasion. Rather, the invasion suppressor signal is mediated through the beta-catenin-binding domain of the E-cadherin cytoplasmic tail but not through the p120ctn-binding domain. beta-catenin depletion also results in invasion suppression. However, alteration in the beta-catenin/TCF transcriptional regulation of target genes is not required for the invasion suppressor activity of E-cadherin, suggesting the involvement of other beta-catenin-binding proteins.
Figures
Figure 1.
A tet-on inducible E-cadherin expression system. (A) MDA-MB-231 and (B) TSU-Pr1 cells. Equivalent micrograms of lysates from stable clones expressing the empty vector control, E-cadherin, E-cadherin–α-catenin, IL2R–cytoplasmic tail, E-cadherinΔp120, and E-cadherinΔβ-catenin; each untreated (−) or treated (+) with 1 μg/ml doxycycline were analyzed by Western blot using a mouse anti–human E-cadherin mAb (Transduction Laboratory). The blot was reprobed with an antiactin antibody as a loading control.
Figure 2.
Schematic diagram of E-cadherin constructs used in this study (adopted from Gottardi et al. [2001] with some modifications). Wild-type E-cadherin is shown at the top. The E-cadherin–α-catenin fusion chimera was designed to mediate adhesion without interacting with β-catenin. Conversely, the IL2R–cytoplasmic tail chimera binds β-catenin but is defective in adhesion. The E-cadherinΔp120 mutant contains point mutations (EED > AAA) that abolish p120ctn binding to E-cadherin and abolish adhesion. The E-cadherinΔβ-catenin mutant has the β-catenin–binding region truncated, it lacks the ability to bind β-catenin, and exhibits little or no adhesive activity.
Figure 3.
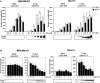
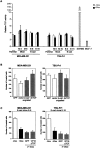
Inhibition of invasion by E-cadherin induction. In vitro Matrigel invasion of tetracycline-regulated MDA-MB-231 and TSU-Pr1 cells was determined as a function of increasing cell numbers and concentration of doxycycline. (A) An increasing number of cells was added to the top compartment of Matrigel filters in the absence (white bars) or presence (black bars) of 1 μg/ml doxycycline. (B) 2 × 105 MDA-MB-231 cells and 105 TUS-Pr1 cells were added onto Matrigel filters in the presence of varying amount of doxycycline (indicated). Values shown are the mean number of cells counted in four fields for replicate transwells from one experiment. Bars represent SD.
Figure 4.
Inhibition of invasion due to expression of the E-cadherin cytoplasmic tail but not increased cell adhesion. (A) Induction of construct expression in stable lines by tet-on system. Stable clones expressing the empty vector control, E-cadherin, E-cadherin–α-catenin, IL2R–cytoplasmic tail, E-cadherinΔp120, or E-cadherinΔβ-catenin were added to the top compartment of Matrigel filters in the absence (white bars) or presence (black bars) of 1 μg/ml doxycycline. (B) Cells were transiently transfected with constructs along with GFP vector to sort expressing cells by flow cytometry. 105 sorted cells were then employed for in vitro Matrigel invasion assays. Values shown are the mean number of cells counted in four fields for replicate transwells from one experiment. Bars represent SD. *P < 0.05, which is considered statistically different from uninduced (white bars) cells.
Figure 5.
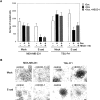
Adhesive properties of cadherin-expressing cell lines. (A and B) Use of a laminar flow adhesion assay and (C and D) use of a cell aggregation assay. (A) MDA-MB-231 and (B) TSU-Pr1 cells expressing E-cadherin and mutant E-cadherin constructs. Micrographs of (C) MDA-MB-231 and (D) TSU-Pr1 cells expressing various constructs in aggregation assays. All cadherin cell lines were induced with 1 μg/ml doxycycline to express a transgene, and the mock control cells were treated with the same level of doxycycline. Bar, 20 μm.
Figure 6.
Treatment with anti–E-cadherin mAb HECD-1 does not revert E-cadherin–mediated invasion suppression. (A) Doxycycline-untreated (white bars) and treated (black bars). Mock- transfected control cells and cells stably expressing E-cadherin were plated on Matrigel filters for invasion assays in the presence (hatched bars) or absence (black bars) of HECD-1 antibody (50 μg/ml). After 24 h, the number of cells traversing the filter was determined. Data shown are the mean number of cells counted in four fields for replicate transwells from one experiment. Bars represent SD. (B) Corresponding control cell dissociation experiment (phase–contrast micrographs) showing inhibition of cell to cell adhesion by HECD-1 (50 μg/ml). Bar, 50 μm.
Figure 7.
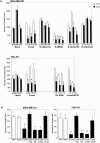
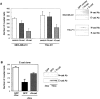
siRNA-mediated depletion of β-catenin suppresses invasion. (A) Western blot of cell lysates from equal protein of corresponding samples with antibodies as indicated. (B) MDA-MB-231 and TSU-Pr1 cells were transfected with β-catenin-siRNA (black bars) or a NS-siRNA (hatched bars) for 1 d and then harvested for invasion assays. Data are expressed as the mean number of cells counted in four fields for replicate transwells from one experiment. Bars represent SD. *P < 0.05, which is considered statistically different from untreated (white bars) cells.
Figure 8.
No role for β-catenin/TCF transcriptional regulation in invasive activity of MDA-MB-231 and TSU-Pr1 cells. (A) No detectable changes in β-catenin/TCF signaling by E-cadherin. Analysis of TCF reporter gene activation in various cell lines. β-catenin/TCF–mediated transcriptional activation was determined by transient transfection of TOPFLASH or FOPFLASH reporter constructs, which contain wild-type or mutant TCF-binding motifs upstream of the firefly luciferase cDNA. APC mutant colorectal cancer cell line SW480 served as a positive control. Stable clones expressing the empty vector control and E-cadherin in the absence (white bars) or presence (black bars) of 1 μg/ml doxycycline were assessed for their ability to modulate TCF-dependent transcription. TCF- mediated transcriptional activity was defined as the ratio of TOPFLASH:FOPFLASH luciferase activities, each corrected internally for Renilla luciferase activities and where no transactivation equals 1. (B) Inhibition of β-catenin/TCF signaling pathway does not suppress invasion. Cells were transiently transfected with GFP alone, an empty vector, a β-catenin/engrailed repressor, and a dominant-negative (dn) TCF and were assessed for in vitro invasion. (C) Constitutively active form of TCF (VP16ΔTCF) does not rescue E-cadherin– mediated invasion suppression. Values shown are the mean number of cells counted in four fields for replicate transwells from one experiment. Bars represent SD.
Figure 9.
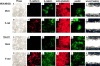
Expression of E-cadherin has no obvious effect on morphology, cytoskeletal organization, or migration of MDA-MB-231 and TSU-Pr1 cells. (A–D) Phase microscopy and indirect immunofluorescence staining of (E–H) E-cadherin (CY3), (I–L) β-catenin (FITC), and (Q–T) paxillin (FITC) in mock and E-cadherin stable cell lines. E-cadherin–transfected cells were treated with 1 μg/ml doxycycline to induce E-cadherin expression, and the control cells were treated with the same level of doxycycline. (M–P) F-actin was visualized after the binding of Texas red–conjugated phalloidin. (U–X) Effect of E-cadherin expression on cell migration. Confluent monolayers of MDA-MB-231 and TSU-Pr1 cultures of cells treated with 1 μg/ml doxycycline to induce expression of E-cadherin were scraped with a pipet tip to create a wound. The mock controls were treated with the same level of doxycycline. Cells were given fresh medium containing doxycycline in the presence of roscovitine (20 μM, to reduce cell growth), cultured for 24 h, and photographed. Bar, 10 μm.
Figure 10.
Effect of N-cadherin on invasion. (A) N-cadherin does not suppress invasion as well as E-cadherin. Parental cells transiently transfected with either N-cadherin or E-cadherin were compared with GFP control cells in invasion assays. (B) N-cadherin promotes invasion of E-cadherin–expressing cells. Doxycycline-induced E-cadherin cells transiently transfected with N-cadherin was compared with GFP controls. Values shown are the mean number of cells counted in four fields for replicate transwells from one experiment. Bars represent SD. Western blot of cell lysates from transient transfection samples before analyzed by FACS® were examined for cadherin expression with antibodies as indicated.
Similar articles
- E-cadherin suppresses cellular transformation by inhibiting beta-catenin signaling in an adhesion-independent manner.
Gottardi CJ, Wong E, Gumbiner BM. Gottardi CJ, et al. J Cell Biol. 2001 May 28;153(5):1049-60. doi: 10.1083/jcb.153.5.1049. J Cell Biol. 2001. PMID: 11381089 Free PMC article. - Truncation of the extracellular region abrogrates cell contact but retains the growth-suppressive activity of E-cadherin.
Sasaki CY, Lin H, Morin PJ, Longo DL. Sasaki CY, et al. Cancer Res. 2000 Dec 15;60(24):7057-65. Cancer Res. 2000. PMID: 11156412 - Autoregulation of E-cadherin expression by cadherin-cadherin interactions: the roles of beta-catenin signaling, Slug, and MAPK.
Conacci-Sorrell M, Simcha I, Ben-Yedidia T, Blechman J, Savagner P, Ben-Ze'ev A. Conacci-Sorrell M, et al. J Cell Biol. 2003 Nov 24;163(4):847-57. doi: 10.1083/jcb.200308162. Epub 2003 Nov 17. J Cell Biol. 2003. PMID: 14623871 Free PMC article. - Alterations of the cadherin-catenin cell adhesion system in cancers.
Kanai Y, Oda T, Shimoyama Y, Ochiai A, Oyama T, Yoshiura K, Akimoto S, Yamada T, Hirohashi S. Kanai Y, et al. Princess Takamatsu Symp. 1994;24:51-62. Princess Takamatsu Symp. 1994. PMID: 8983063 Review. - The role of the E-cadherin/catenin complex in gastrointestinal cancer.
Debruyne P, Vermeulen S, Mareel M. Debruyne P, et al. Acta Gastroenterol Belg. 1999 Oct-Dec;62(4):393-402. Acta Gastroenterol Belg. 1999. PMID: 10692769 Review.
Cited by
- Molecular mechanism of pancreatic cancer--understanding proliferation, invasion, and metastasis.
Mihaljevic AL, Michalski CW, Friess H, Kleeff J. Mihaljevic AL, et al. Langenbecks Arch Surg. 2010 Apr;395(4):295-308. doi: 10.1007/s00423-010-0622-5. Epub 2010 Mar 18. Langenbecks Arch Surg. 2010. PMID: 20237938 Review. - Dual Src Kinase/Pretubulin Inhibitor KX-01, Sensitizes ERα-negative Breast Cancers to Tamoxifen through ERα Reexpression.
Anbalagan M, Sheng M, Fleischer B, Zhang Y, Gao Y, Hoang V, Matossian M, Burks HE, Burow ME, Collins-Burow BM, Hangauer D, Rowan BG. Anbalagan M, et al. Mol Cancer Res. 2017 Nov;15(11):1491-1502. doi: 10.1158/1541-7786.MCR-16-0297-T. Epub 2017 Jul 27. Mol Cancer Res. 2017. PMID: 28751463 Free PMC article. - Molecular mechanisms controlling E-cadherin expression in breast cancer.
Baranwal S, Alahari SK. Baranwal S, et al. Biochem Biophys Res Commun. 2009 Jun 19;384(1):6-11. doi: 10.1016/j.bbrc.2009.04.051. Epub 2009 Apr 18. Biochem Biophys Res Commun. 2009. PMID: 19379710 Free PMC article. Review. - Guidance of Signaling Activations by Cadherins and Integrins in Epithelial Ovarian Cancer Cells.
Roggiani F, Mezzanzanica D, Rea K, Tomassetti A. Roggiani F, et al. Int J Mol Sci. 2016 Aug 23;17(9):1387. doi: 10.3390/ijms17091387. Int J Mol Sci. 2016. PMID: 27563880 Free PMC article. Review. - A Novel N-Sulfonylamidine-Based Derivative Inhibits Proliferation, Migration, and Invasion in Human Colorectal Cancer Cells by Suppressing Wnt/β-Catenin Signaling Pathway.
Zhao X, Han Z, Ma J, Jiang S, Li X. Zhao X, et al. Pharmaceutics. 2021 May 3;13(5):651. doi: 10.3390/pharmaceutics13050651. Pharmaceutics. 2021. PMID: 34063618 Free PMC article.
References
- Albini, A. 1998. Tumor and endothelial cell invasion of basement membranes. The matrigel chemoinvasion assay as a tool for dissecting molecular mechanisms. Pathol. Oncol. Res. 4:230–241. - PubMed
- Anastasiadis, P.Z., S.Y. Moon, M.A. Thoreson, D.J. Mariner, H.C. Crawford, Y. Zheng, and A.B. Reynolds. 2000. Inhibition of RhoA by p120 catenin. Nat. Cell Biol. 2:637–644. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous