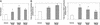Pronounced reduction in adenoma recurrence associated with aspirin use and a polymorphism in the ornithine decarboxylase gene - PubMed (original) (raw)
Pronounced reduction in adenoma recurrence associated with aspirin use and a polymorphism in the ornithine decarboxylase gene
Maria Elena Martinez et al. Proc Natl Acad Sci U S A. 2003.
Abstract
Most sporadic colon adenomas acquire mutations in the adenomatous polyposis coli gene (APC) and show defects in APC-dependent signaling. APC influences the expression of several genes, including the c-myc oncogene and its antagonist Mad1. Ornithine decarboxylase (ODC), the first enzyme in polyamine synthesis, is a transcriptional target of c-myc and a modifier of APC-dependent tumorigenesis. A single-nucleotide polymorphism exists in intron 1 of the human ODC gene, which lies between two myc-binding domains. This region is known to affect ODC transcription, but no data exist on the relationship of this polymorphism to risk of colorectal neoplasia in humans. We show that individuals homozygous for the minor ODC A-allele who reported using aspirin are approximately 0.10 times as likely to have an adenoma recurrence as non-aspirin users homozygous for the major G-allele. Mad1 selectively suppressed the activity of the ODC promoter containing the A-allele, but not the G-allele, in a human colon cancer-derived cell line (HT29). Aspirin (>or=10 microM) did not affect ODC allele-specific promoter activity but did activate polyamine catabolism and lower polyamine content in HT29 cells. We propose that the ODC polymorphism and aspirin act independently to reduce the risk of adenoma recurrence by suppressing synthesis and activating catabolism, respectively, of colonic mucosal polyamines. These findings confirm the hypothesis that the ODC polymorphism is a genetic marker for colon cancer risk, and support the use of ODC inhibitors and aspirin, or other nonsteroidal antiinflammatory drugs (NSAIDs), in combination as a strategy for colon cancer prevention.
Figures
Scheme 1.
Fig. 1.
Odds ratios for adenoma recurrence as a function of ODC genotype and aspirin use. Results of logistic regression models that control for age, gender, and number of colonoscopies are shown (95% confidence intervals). Individuals homozygous for the G-allele and who reported no aspirin use are the reference (Ref) group. The number of individuals who recurred over the total in each group among non-aspirin users are 137/266 (51.5%), 100/182 (55.0%), and 13/31 (41.9%) for GG, AG, and AA variants, respectively; the corresponding figures among aspirin users are 55/116 (47.4%), 34/82 (41.5%), and 2/11 (18.2%).
Fig. 2.
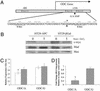
A single-nucleotide polymorphism (SNP) in the ODC promoter and its significance for _APC_-dependent ODC promoter activity. (A) ODC gene structure. There are three consensus-binding sites for the c-myc:Max and Mad1:Max heterodimers within the ODC gene. These sites, called E-boxes, have the consensus sequence CACGTG. E-box (1) is located in the 5′ flanking region of the gene, and E-box (2) and E-box (3) are separated by 28 nt in the first intron. The G/A SNP (*) is positioned between the two E-boxes in intron 1 at +316. Also marked in this diagram are the nearby CpG sequences. (B) Effect of wild-type APC expression on the level of three E-box transcription factors. HT29-APC and control HT29-β-gal cells were treated with 300 μM ZnCl2 for 5 h. Whole-cell lysates from untreated and treated cells were run on an SDS/PAGE gel and Western blots were performed for c-myc, Mad, and Max, as described in Methods. (C) Effect of wild-type APC expression on allelespecific ODC promoter activity. HT29-APC (open bars) and HT29-β-gal (filled bars) cells were transfected with either pGL3-ODC/A or pGL3-ODC/G, as described in Methods. Transfected cultures were then treated with or without 300 μM ZnCl2 for an additional 24 h and harvested, and lysates were analyzed for luciferase activity. Results are presented as the ratios of mean values ± standard deviations of triplicate measurements from cultures treated with ZnCl2 divided by those from similarly transfected cells not treated with ZnCl2. The result is an average of three experiments. Bars indicate standard deviations. *, Statistically significant effects (P < 0.05). (D) Effect of Mad1 expression on allele-specific ODC promoter activity. By using HT29 cells, either pGL3-ODC/A or pGL3-ODC/G was cotransfected with pcDNA3.1 or pcDNA-Mad1 in a molar ratio of 3:1. The cells were harvested after 48 h, and lysates were analyzed for luciferase activity. Experiments were performed in triplicate. Bars indicate standard deviation. *, Statistically significant effects (P < 0.05).
Fig. 3.
Aspirin induces SSAT promoter, RNA, and enzyme activity in HT29 cells. (A) SSAT promoter activity. HT29 cells were transfected with reporter SSAT-Luc along with pCMV-β-gal plasmid. Relative luciferase units (RLU) were calculated after normalizing to the protein and β-gal activities in the cell lysates. Fold induction was calculated after dividing the RLU of sample by the RLU of vehicle. Bars indicate standard deviation. *, Statistically significant difference (P < 0.05) compared with vehicle. (B) SSAT RNA. HT29 cells were seeded and grown for 24 h. The cells were then treated with indicated amounts of aspirin for 48 h and harvested, and total RNA was extracted. Total RNA was analyzed by probing for SSAT and GAPDH. Fold induction was calculated by dividing normalized sample values to the GAPDH control. Bars indicate standard deviation. *, Statistically significant difference (P < 0.05) compared with vehicle. (C) SSAT enzyme activity. HT29 cells were grown overnight and then treated with either various concentrations of aspirin or its vehicle for 24 h. Cells were harvested and SSAT enzyme activity was measured as described in Methods. The results are an average from three different experiments. Bars indicate standard deviation. *, Statistically significant difference (P < 0.05) compared with vehicle.
Fig. 4.
Decrease in polyamine levels in HT29 cells caused by aspirin. Cells were treated for 48 h with either 20 or 100μM aspirin or its vehicle (ethanol). Polyamine levels were normalized to the protein in the samples. Fold changes were then calculated after dividing the polyamine level in the sample by the polyamine level in the vehicle control and plotted. Bars indicate standard deviations. *, Statistically significant difference (P < 0.05) compared with vehicle.
Fig. 5.
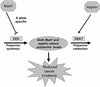
Model depicting actions of ODC polymorphism and aspirin on polyamine metabolism, and the consequences for adenoma recurrence. Before acquisition of somatic APC mutations, colonic cells in individuals with the minor A-allele would have reduced polyamine synthesis due to selective suppression of ODC promoter activity by Mad1. Aspirin would act, in an ODC allele-independent manner, to reduce further polyamine levels by activating the transcription of SSAT, thereby increasing polyamine catabolism and export. Cancer risk would be directly related to colonic mucosal polyamine contents, being decreased by decreased polyamine levels.
Similar articles
- APC-dependent regulation of ornithine decarboxylase in human colon tumor cells.
Fultz KE, Gerner EW. Fultz KE, et al. Mol Carcinog. 2002 May;34(1):10-8. doi: 10.1002/mc.10043. Mol Carcinog. 2002. PMID: 12112318 - Ornithine decarboxylase polymorphism modification of response to aspirin treatment for colorectal adenoma prevention.
Barry EL, Baron JA, Bhat S, Grau MV, Burke CA, Sandler RS, Ahnen DJ, Haile RW, O'Brien TG. Barry EL, et al. J Natl Cancer Inst. 2006 Oct 18;98(20):1494-500. doi: 10.1093/jnci/djj398. J Natl Cancer Inst. 2006. PMID: 17047198 - Associations of a polymorphism in the ornithine decarboxylase gene with colorectal cancer survival.
Zell JA, Ziogas A, Ignatenko N, Honda J, Qu N, Bobbs AS, Neuhausen SL, Gerner EW, Anton-Culver H. Zell JA, et al. Clin Cancer Res. 2009 Oct 1;15(19):6208-16. doi: 10.1158/1078-0432.CCR-09-0592. Epub 2009 Sep 29. Clin Cancer Res. 2009. PMID: 19789310 Free PMC article. - A comprehensive strategy to combat colon cancer targeting the adenomatous polyposis coli tumor suppressor gene.
Gerner EW, Ignatenko NA, Lance P, Hurley LH. Gerner EW, et al. Ann N Y Acad Sci. 2005 Nov;1059:97-105. doi: 10.1196/annals.1339.033. Ann N Y Acad Sci. 2005. PMID: 16382048 Review. - Polyamines as mediators of APC-dependent intestinal carcinogenesis and cancer chemoprevention.
Rial NS, Meyskens FL, Gerner EW. Rial NS, et al. Essays Biochem. 2009 Nov 4;46:111-24. doi: 10.1042/bse0460008. Essays Biochem. 2009. PMID: 20095973 Free PMC article. Review.
Cited by
- Current concepts in colorectal cancer prevention.
Thompson PA, Gerner EW. Thompson PA, et al. Expert Rev Gastroenterol Hepatol. 2009 Aug;3(4):369-82. doi: 10.1586/egh.09.28. Expert Rev Gastroenterol Hepatol. 2009. PMID: 19673624 Free PMC article. Review. - Indomethacin Induces Spermidine/Spermine-N1-Acetyltransferase-1 via the Nucleolin-CDK1 Axis and Synergizes with the Polyamine Oxidase Inhibitor Methoctramine in Lung Cancer Cells.
Buelvas N, Ugarte-Vio I, Asencio-Leal L, Muñoz-Uribe M, Martin-Martin A, Rojas-Fernández A, Jara JA, Tapia JC, Arias ME, López-Muñoz RA. Buelvas N, et al. Biomolecules. 2023 Sep 12;13(9):1383. doi: 10.3390/biom13091383. Biomolecules. 2023. PMID: 37759783 Free PMC article. - Pharmacological and dietary prevention for colorectal cancer.
Nolfo F, Rametta S, Marventano S, Grosso G, Mistretta A, Drago F, Gangi S, Basile F, Biondi A. Nolfo F, et al. BMC Surg. 2013;13 Suppl 2(Suppl 2):S16. doi: 10.1186/1471-2482-13-S2-S16. Epub 2013 Oct 8. BMC Surg. 2013. PMID: 24267792 Free PMC article. Review. - Molecular targets for cancer chemoprevention.
William WN Jr, Heymach JV, Kim ES, Lippman SM. William WN Jr, et al. Nat Rev Drug Discov. 2009 Mar;8(3):213-25. doi: 10.1038/nrd2663. Nat Rev Drug Discov. 2009. PMID: 19247304 Review. - Alpha-Difluoromethylornithine, an Irreversible Inhibitor of Polyamine Biosynthesis, as a Therapeutic Strategy against Hyperproliferative and Infectious Diseases.
LoGiudice N, Le L, Abuan I, Leizorek Y, Roberts SC. LoGiudice N, et al. Med Sci (Basel). 2018 Feb 8;6(1):12. doi: 10.3390/medsci6010012. Med Sci (Basel). 2018. PMID: 29419804 Free PMC article. Review.
References
- Morson, B. C. (1974) Cancer 34, Suppl., 845-849. - PubMed
- Muto, T., Bussey, H. J. & Morson, B. C. (1975) Cancer 36, 2251-2270. - PubMed
- Fearon, E. R. & Vogelstein, B. (1990) Cell 61, 759-767. - PubMed
- Leslie, A., Carey, F. A., Pratt, N. R. & Steele, R. J. C. (2002) Br. J. Surg. 89, 845-865. - PubMed
- Nishisho, I., Nakamura, Y., Miyoshi, Y., Ando, H., Horii, A., Koyama, K., Utsunomiya, J., Baba, S. & Hedge, P. (1991) Science 253, 665-659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA023074/CA/NCI NIH HHS/United States
- CA-95060/CA/NCI NIH HHS/United States
- CA-41108/CA/NCI NIH HHS/United States
- KO1 CA79069-10/CA/NCI NIH HHS/United States
- P01 CA041108/CA/NCI NIH HHS/United States
- P50 CA095060/CA/NCI NIH HHS/United States
- CA-23074/CA/NCI NIH HHS/United States
- CA-72008/CA/NCI NIH HHS/United States
- P01 CA072008/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials