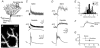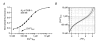Mutational analysis of dendritic Ca2+ kinetics in rodent Purkinje cells: role of parvalbumin and calbindin D28k - PubMed (original) (raw)
Mutational analysis of dendritic Ca2+ kinetics in rodent Purkinje cells: role of parvalbumin and calbindin D28k
Hartmut Schmidt et al. J Physiol. 2003.
Abstract
The mechanisms governing the kinetics of climbing fibre-mediated Ca2+ transients in spiny dendrites of cerebellar Purkinje cells (PCs) were quantified with high-resolution confocal Ca2+ imaging. Ca2+ dynamics in parvalbumin (PV-/-) and parvalbumin/calbindin D28k null-mutant (PV/CB-/-) mice were compared with responses in wild-type (WT) animals. In the WT, Ca2+ transients in dendritic shafts were characterised by double exponential decay kinetics that were not due to buffered Ca2+ diffusion or saturation of the indicator dye. Ca2+ transients in PV-/- PCs reached the same peak amplitude as in the WT but the biphasic nature of the decay was less pronounced, an effect that could be attributed to PV's slow binding kinetics. In contrast, peak amplitudes in PV/CB-/- PCs were about two times higher than in the WT and the decay became nearly monophasic. Numerical simulations indicate that the residual deviation from a single exponential decay in PV/CB-/- is due to saturation of the Ca2+ indicator dye. Furthermore, the simulations imply that the effect of uncharacterised endogenous Ca2+ binding proteins is negligible, that buffered diffusion and dye saturation significantly affects spineous Ca2+ transients but not those in the dendritic shafts, and that neither CB nor PV undergoes saturation in spines or dendrites during climbing fibre-evoked Ca2+ transients. Calbindin's medium-affinity binding sites are fast enough to reduce the peak amplitude of the Ca2+ signal. However, similar to PV, delayed binding by CB leads to biphasic Ca2+ decay kinetics. Our results suggest that the distinct kinetics of PV and CB underlie the biphasic kinetics of synaptically evoked Ca2+ transients in dendritic shafts of PCs.
Figures
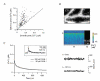
Figure 1. Climbing fibre-mediated Ca2+ transients in spines and dendrites of cerebellar Purkinje cells
A, schematic drawing of the experimental arrangement. The cells were loaded with the calcium indicator dye Oregon Green BAPTA-1 (OGB-1, 200 μM) via a somatic patch pipette. The climbing fibre (grey), which contacts the Purkinje cell on the proximal dendrites, was stimulated with an extracellular electrode (left) located in the granule cell layer. Ca2+ transients were recorded in the distal dendritic branchlets. B, contrast-enhanced confocal image of distal dendrites and adjacent spines. The arrows indicate the spine and the dendritic region from which the Ca2+-dependent fluorescence signals were recorded in the point-scan mode (see C and D). C, fluorescence transients (Δ_F_/_F_0) in the spine and the dendrite (upper and middle trace, respectively) evoked by CF activation. The CF-mediated EPSP, with the stimulation artefact blanked, is shown on an expanded time scale in the bottom trace. The arrowhead denotes the time point of synaptic stimulation. D, rising phase of the transients shown in C plotted on the same time scale as the EPSP. The continuous lines represent fits (see eqn (1)) to the transients The dashed line indicates the starting point (_t_0) of the dendritic transient estimated by the fit. E, temporal distribution of the peak amplitude of the first and second spike of the CF-EPSP, as well as the start of the second spike (‘1st min’) relative to _t_0 of the fluorescence transients. The continuous lines represent Gaussian fits to the distributions (_t_0 = -1.3, -0.1 and 1.2 ms, half-width = 0.8, 0.6 and 1.2 ms for the first peak, the first minimum and the second peak, respectively; n = 43 from five cells from five mice). Note that _t_0 correlates best with the start of the second spike. F, comparison of the fitted rising phases of the fluorescence transients shown in D. The responses were normalised to their peak amplitudes. The arrows indicate the time points at which the peak amplitudes were reached. G, peak amplitudes (spine: 1.84, 1.50-2.20; dendrite 1.17, 0.94-1.42; median and IQR) of the fluorescence transients in spines and dendrites (n = 32 and 24, respectively) plotted against the corresponding time-to-peak values (spine: 11.4 ± 3.4 ms; dendrite: 15.5 ± 4.8 ms; mean ± S.D.). Spine transients were significantly larger and reached their maximum earlier than in the dendritic responses (P < 0.001).
Figure 6. Dendritic decay kinetics in parvalbumin/calbindin D28k null-mutant (PV/CB−/−) mice
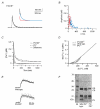
A, example trace. The continuous line represents a double exponential fit to the decay. The inset shows the fast and slow components (red and blue traces, respectively). The fit yielded a fast component of 120 nM with a τfast of 19 ms and a slow component of 220 nM with a τslow of 135 ms. B, data from double exponential decays from 62 dendrites from six cells from six mice. The fast and slow components are shown in red and blue, respectively. C, calculated median decay in dendrites of PV/CB−/− mice (dotted trace) compared with the median response in PV mice (dashed trace). The grey area represents the integral of the arithmetic difference between the two decays. D, [Ca2+]i dependence of the [Ca2+]i decay rates in WT (continuous line), PV−/− (dashed line) and PV/CB−/− (dotted line) dendrites. The decay rates were calculated from the corresponding median dendritic decays. E, examples of point-scan recordings showing the rising phase of the Ca2+ transients in WT and PV/CB−/− animals. F, 45Ca2+-overlay assay of cerebella from WT, PV−/− and PV/CB−/− mice. ‘CR’ denotes the calretinin bands and ‘M’ the lane containing marker proteins. Note that no PV or PV/CB staining is observed in the mutant mice and that no upregulation of other CaBPs occurred.
Figure 2. Quantification of the fluorescence signals
A, fluorescence intensity of OGB-1 in the pipette solution buffered to various free calcium concentrations ([Ca2+]free). Values were normalised to the intensity obtained at ≈20 μM [Ca2+]free. Data points represent means ± S.D. from five independent cuvette calibrations. The continuous line represents a least-squares fit that yielded a K_D of 325 nM (see eqn (2)). The dashed line represents the assumed intracellular resting calcium concentration ([Ca2+]rest) under our experimental conditions. B, conversion (black line) of Δ_F/_F_0 values to [Ca2+]free for a [Ca2+]rest of 45 nM and the associated F_0. The grey lines represent the error range if the cellular [Ca2+]rest deviates by ± 10 % from 45 nM. Based on the calibration, fluorescence transients that exceeded 2.5 Δ_F/_F_0 were discarded.
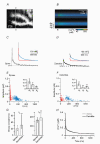
Figure 3. Double exponential decay kinetics of Ca2+ transients in spines and dendrites from wild-type (WT) mice
A, confocal image of the dendritic area in which the line-scan recording shown in _B_-D was performed. The white line and the open triangles indicate the position of the single line that was analysed. B, colour-coded image of the line-scan (i.e. y-t) recording from the region outlined in A. The brackets denote the regions of interest from which the dendritic (‘de’), spine (‘sp’) and background (‘bg’) signals were obtained. The time point of CF stimulation is indicated by the arrowhead. C and D, waveform of the Ca2+ transients in the spine (C) and the dendrite (D). The continuous lines represent double exponential fits to the decay; the dashed lines represent the assumed resting [Ca2+]i of 45 nM. The insets show the fits with their corresponding fast and slow components (red and blue traces, respectively) superimposed. In the spine the fit yielded a fast component of 255 nM with a decay time constant (τfast) of 17 ms and a slow component of 135 nM with a τslow of 330 ms. The corresponding dendritic values were 70 nM and 15 ms (fast component) and 110 nM and 460 ms (slow component). E and F, data for double exponential decays from 252 spines (E) and 131 dendrites (F) from 19 cells from 18 animals. The fast and slow components are plotted in red and blue, respectively. The insets show the median and IQR values of the total amplitude (_A_t) as well as the amplitudes of the fast (_A_f) and slow (_A_s) components. **Statistically different from spines (P < 0.005). G, corresponding values of τfast and τslow (median and IQR). H, calculated median decay in spines (grey trace) and dendrites (black trace).
Figure 4. Influence of Ca2+ diffusion and the indicator dye on the decay kinetics
A, plot of the peak amplitude of Ca2+ responses in spines versus the peak amplitude in the corresponding dendrites. Note that the dendritic peak amplitude was in almost all cases smaller than in the adjacent spines. B, upper panel: dendritic segment in which the line-scan recording shown below was performed. The grey line and open triangles mark the region from which the fluorescence was sampled. Middle panel: colour-coded image of the line-scan (x-t) recording from the region outlined above. The brackets denote the regions of interest from which the dendritic (‘de’) and background (‘bg’) signals were obtained. The arrowhead indicates the time point of CF stimulation. Lower panel: corresponding Ca2+ levels at the peak of the CF response (‘t = 0 ms’) and 100 ms later. The continuous lines represent linear fits to the data. At both time points the slope of the fits was < 1 nM μm−1. C, comparison of the median dendritic decays recorded with a pipette concentration of OGB-1 of 200 μM (same as in Fig. 3_H_) and 50 μM (n = 69, five cells, three animals, _A_f = 131 nM, _A_s = 100 nM, τfast = 36 ms, τslow = 378 ms). The inset shows an example trace recorded with 50 μM OGB-1. A double exponential fit (continuous line) was required to faithfully describe the decay (_A_f = 180 nM, _A_s = 108 nM, τfast = 16 ms, τslow = 333 ms).
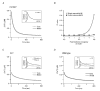
Figure 5. Dendritic decay kinetics in parvalbumin null-mutant (PV−/−) mice
A, example of a dendritic recording. The continuous line represents a double exponential fit to the decay. The inset shows the fit with its corresponding fast (70 nM and 28 ms, red trace) and slow components (90 nM and 280 ms, blue trace) superimposed. B, data of double exponential decays from 48 dendrites from five cells from five mice. The fast and slow components are plotted in red and blue, respectively. C, calculated median decay in dendrites of PV−/− mice (dashed trace) compared with the median response in WT mice (continuous trace). The grey area represents the integral of the arithmetic difference between the two decays. The bracket denotes the time interval in which a significant difference between the WT and PV−/− transients occurred (t test, P < 0.025). D, comparison of the [Ca2+]i dependence of the median dendritic decay rates (-d[Ca2+]i / d_t_; calculated from C) between WT (continuous line) and PV−/− (dashed line).
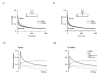
Figure 7. Single-compartment model of Ca2+ transients in dendritic shafts
A, median dendritic decay kinetics (dotted black line) and corresponding simulated decay of the [Ca2+]i reported by OGB-1 (grey line) from PV/CB−/− PCs. The kinetic model incorporated a Gaussian Ca2+ influx, OGB-1 and a Ca2+ extrusion mechanism that was linear in the range of the Ca2+ transient (see Methods and Table 2 for details), reflecting the situation in the double mutant. The inset shows the fractional Ca2+ occupancy of OGB-1 during the simulated Ca2+ transient. B, χ2 values of single and double exponential fits (open and filled symbols, respectively) to increasingly larger Ca2+ transients simulated with the model used in A. The lines represent polynomial fits to the χ2 values. Note that double exponential fits are required to faithfully describe the decay kinetics once the peak Ca2+ concentration reaches the _K_D of OGB-1 (>50 % occupancy). C, the median dendritic decay in PV−/− PCs (black dashed line) was modelled (grey line) by including 40 μM CB with two high- and two medium-affinity binding sites into the simulation described in A. The inset shows the fractional Ca2+ occupancy of OGB-1 and CB's high- and medium-affinity binding sites. D, the median dendritic decay in WT PCs (black line) was modelled (grey line) by including 40 μM PV in the simulation described in C. The inset shows the corresponding fractional Ca2+ occupancy of OGB-1, of CB's bindings sites and of PV. The simulations of the PV/CB−/− transients required a two-fold higher extrusion rate than those of the PV−/− and WT transients (see Results for details).
Figure 8. Compartmental model of spino-dendritic Ca2+ dynamics
A, measured and simulated Ca2+ decay kinetics (black and grey traces, respectively) in spines and dendrites of WT PCs. For the simulation, the diffusional coupling between the spineous and the dendritic compartment was not allowed to occur. Note that the simulation faithfully reproduces the measured dendritic transient (same result as shown in Fig. 7_D_), but fails to reproduce the spine transient. B, same as A but with diffusional coupling taking place on Ca2+ and all buffer species (Ca2+- and Mg2+-bound as well as free OGB-1, CB and PV) were allowed to diffuse between the spine and the dendrite (see Methods and Table 2 for details). Note that diffusional coupling resulted in a more reliable simulation of the spine transient but did not significantly affect the dendritic decay. C, fractional occupancy of OGB-1, PV and CB's binding sites in the spine (C1) and the dendritic shaft (C2) coupled by diffusion (see B). The only buffer that undergoes saturation (fractional occupancy > 50 %) is the spineous OGB-1.
Similar articles
- Spino-dendritic cross-talk in rodent Purkinje neurons mediated by endogenous Ca2+-binding proteins.
Schmidt H, Kunerth S, Wilms C, Strotmann R, Eilers J. Schmidt H, et al. J Physiol. 2007 Jun 1;581(Pt 2):619-29. doi: 10.1113/jphysiol.2007.127860. Epub 2007 Mar 8. J Physiol. 2007. PMID: 17347272 Free PMC article. - The role of parvalbumin and calbindin D28k in experimental scrapie.
Voigtländer T, Unterberger U, Guentchev M, Schwaller B, Celio MR, Meyer M, Budka H. Voigtländer T, et al. Neuropathol Appl Neurobiol. 2008 Aug;34(4):435-45. doi: 10.1111/j.1365-2990.2007.00902.x. Epub 2007 Nov 25. Neuropathol Appl Neurobiol. 2008. PMID: 18005331 - Alterations in Purkinje cell spines of calbindin D-28 k and parvalbumin knock-out mice.
Vecellio M, Schwaller B, Meyer M, Hunziker W, Celio MR. Vecellio M, et al. Eur J Neurosci. 2000 Mar;12(3):945-54. doi: 10.1046/j.1460-9568.2000.00986.x. Eur J Neurosci. 2000. PMID: 10762324 - A Ca(2+)-binding protein with numerous roles and uses: parvalbumin in molecular biology and physiology.
Arif SH. Arif SH. Bioessays. 2009 Apr;31(4):410-21. doi: 10.1002/bies.200800170. Bioessays. 2009. PMID: 19274659 Review.
Cited by
- Is Purkinje Neuron Hyperpolarisation Important for Cerebellar Synaptic Plasticity? A Retrospective and Prospective Analysis.
Canepari M. Canepari M. Cerebellum. 2020 Dec;19(6):869-878. doi: 10.1007/s12311-020-01164-0. Cerebellum. 2020. PMID: 32654026 Review. - Cholecystokinin Activation of Cholecystokinin 1 Receptors: a Purkinje Cell Neuroprotective Pathway.
Orr HT. Orr HT. Cerebellum. 2023 Aug;22(4):756-760. doi: 10.1007/s12311-022-01428-x. Epub 2022 Jun 23. Cerebellum. 2023. PMID: 35733029 Free PMC article. Review. - Effects of calretinin on Ca2+ signals in cerebellar granule cells: implications of cooperative Ca2+ binding.
Saftenku EÈ. Saftenku EÈ. Cerebellum. 2012 Mar;11(1):102-20. doi: 10.1007/s12311-011-0263-4. Cerebellum. 2012. PMID: 21394464 - Binding of Filamentous Actin to CaMKII as Potential Regulation Mechanism of Bidirectional Synaptic Plasticity by β CaMKII in Cerebellar Purkinje Cells.
Pinto TM, Schilstra MJ, Roque AC, Steuber V. Pinto TM, et al. Sci Rep. 2020 Jun 2;10(1):9019. doi: 10.1038/s41598-020-65870-9. Sci Rep. 2020. PMID: 32488204 Free PMC article. - The absence of the calcium-buffering protein calbindin is associated with faster age-related decline in hippocampal metabolism.
Moreno H, Burghardt NS, Vela-Duarte D, Masciotti J, Hua F, Fenton AA, Schwaller B, Small SA. Moreno H, et al. Hippocampus. 2012 May;22(5):1107-20. doi: 10.1002/hipo.20957. Epub 2011 May 31. Hippocampus. 2012. PMID: 21630373 Free PMC article.
References
- Allbriton NL, Meyer T, Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 1992;258:1812–1815. - PubMed
- Baimbridge KG, Celio MR, Rogers JH. Calcium-binding proteins in the nervous system. Trends Neurosci. 1992;15:303–308. - PubMed
- Berg H. Random Walks in Biology. 2. Princeton: Princeton University Press; 1993.
- Berggård T, Miron S, Önnerfjord P, Thulin E, Åkerfeldt KS, Enghild JJ, Akke M, Linse S. Calbindin D28k exhibits properties characteristic of a Ca2+ sensor. J Biol Chem. 2002;277:16662–16672. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous