Fibroblasts isolated from normal lungs and those with idiopathic pulmonary fibrosis differ in interleukin-6/gp130-mediated cell signaling and proliferation - PubMed (original) (raw)
Fibroblasts isolated from normal lungs and those with idiopathic pulmonary fibrosis differ in interleukin-6/gp130-mediated cell signaling and proliferation
Yuben P Moodley et al. Am J Pathol. 2003 Jul.
Abstract
Interleukin (IL)-6 and IL-11 are elevated in a variety of lung conditions and may impact on repair mechanisms in chronic inflammatory disorders. However, the mechanisms by which these cytokines influence fibroblast proliferation in normal and disease states have not been previously addressed. We examined the effect of these cytokines on proliferation and cell-cycle kinetics of primary human lung fibroblasts obtained from normal patients and patients with idiopathic pulmonary fibrosis (IPF). IL-6 inhibited the proliferation of normal fibroblasts due to the sustained phosphorylation of STAT-3 and production of the cyclin-dependent kinase inhibitor p19(INK4D). In contrast IL-6 was mitogenic for IPF fibroblasts due to the sustained activation of MAPK, which in turn inhibited the production of p27(Kip1), allowing activation of cyclin D(1) and hyperphosphorylation of retinoblastoma protein. IL-11 was mitogenic for both normal and IPF fibroblasts. These results provide strong evidence for a fundamental abnormality in a cytokine-signaling pathway, as opposed to alterations in cytokine production, in the pathogenesis of IPF.
Figures
Figure 1.
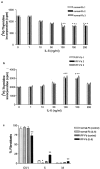
IL-6 inhibits proliferation of normal Fb but is a mitogen for IPF-Fb. Normal Fb (a) or IPF-Fb (b) were incubated with increasing concentrations of IL-6 for 24 hours and proliferation was assessed by incorporation of [3H]-thymidine as described in Materials and Methods. *, P < 0.01 compared with serum-free control (·). c: Exposure to IL-6 caused cell-cycle arrest of normal Fb in G0/1 (black bars), whereas in IPF-Fb, IL-6 induced a significant progression of cells into S phase (striped bars). *, P < 0.01 compared with serum-free normal Fb; **, P < 0.01 compared with serum-free IPF-Fb. Data shown represent mean ± SEM of four experiments performed in triplicate.
Figure 2.
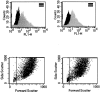
Expression of gp130 is not different between normal Fb and IPF-Fb. Normal Fb (left) or IPF-Fb (right) were permeabilized and incubated with antibodies against gp130 and expression assessed by flow cytometry as described in Materials and Methods. Mean fluorescence intensity values were 52 ± 5 for normal Fb and 58 ± 6 for IPF-Fb. Data shown are representative of each of three normal and three IPF-Fb lines.
Figure 3.
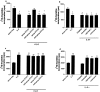
The inhibitory effect of IL-6 on normal Fb is STAT-3-dependent whereas the mitogenic effect on IPF-Fb is ERK-dependent. Normal Fb (a, b) or IPF-Fb (c, d) were incubated with ASON against STAT-3 or STAT 5a/b (a, c) or pharmacological inhibitors to signaling pathways (b, d) and proliferation was assessed by incorporation of [3H]-thymidine as described in Materials and Methods. Data shown are mean ± SEM of four experiments conducted in triplicate. *, P < 0.01 compared with serum-free normal Fb; **, P < 0.01 compared with serum free IPF-Fb.
Figure 4.
IL-6 induces the STAT-3-dependent expression the cdk inhibitor p19INK4D in normal Fb. a: Cells were exposed to IL-6 (100 ng/ml) in the presence or absence of ASON to STAT-3 and the expression of p19INK4D, cyclin D1, cyclin E1, and retinoblastoma protein assessed by Western blot analysis as described in Materials and Methods. The figure is representative of three separate experiments. b: Control blots confirming the specificity of STAT 3 phosphorylation induced by IL-6, but not IL-11. The effect of IL-6 on STAT 3 is blocked by ASON to STAT 3, but not sense oligonucleotides or ASON against STAT 5a/b. c: Control blot showing that exposure to IL-6 for 24 hours does not induce phosphorylation of ERK 1/2 above that seen in unstimulated control cells.
Figure 5.
IL-6 inhibits the expression of cdk inhibitor p27Kip1 in IPF-Fb. a: Cells incubated in serum-free media were exposed to IL-6 (100 ng/ml) in the presence or absence of PD98059. Lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and the expression of p27Kip1, cyclin D1, cyclin E1, and phosphorylated retinoblastoma protein assessed by Western blot analysis as described in Materials and Methods. The figure is representative of three separate experiments. b: Control blots confirming the specificity of STAT 3 phosphorylation induced by IL-6, but not IL-11. The effect of IL-6 on STAT 3 is blocked by ASON to STAT 3, but not sense oligonucleotides or ASON against STAT 5a/b. c: Control blot showing that exposure to IL-6 for 24 hours induces phosphorylation of ERK 1/2 compared to unstimulated control cells. Phosphorylation of ERK 1/2 is reduced to basal levels after exposure to PD98059 (50 μmol/L).
Figure 6.
Sustained STAT-3 signaling in normal Fb is replaced by sustained ERK signaling in CFF-Fb in response to IL-6. Normal Fb (a) or IPF-Fb (b) were exposed to IL-6 (100 ng/ml) for periods of time up to 24 hours. Lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and the duration of phosphorylation (activation) of ERK 1/2 (top) or STAT-3 (bottom) assessed by Western blot analysis. The figure is representative of three separate experiments.
Figure 7.
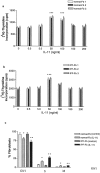
IL-11 is a mitogen for both normal Fb and IPF-Fb. Normal Fb (a) or IPF-Fb (b) were incubated with increasing concentrations of IL-11 for 24 hours and proliferation assessed by incorporation of [3H]-thymidine as described in Materials and Methods. *, P < 0.01 compared with serum-free control (·). *, P < 0.01 compared with serum-free normal Fb, **, P < 0.01 compared with serum-free IPF-Fb. c: Exposure to IL-11 induced a significant progression of both normal Fb (white bars) and IPF-Fb (striped bars) into S phase. Data shown represent mean ± SEM of four experiments performed in triplicate.
Figure 8.
The mitogenic effects of IL-11 on normal Fb and IPF-Fb are ERK-dependent. Normal Fb (a, b) or IPF-Fb (c, d) were incubated with IL-11 (50 ng/ml) in the presence or absence of ASON against STAT-3 or STAT 5a/b (a, c) or pharmacological inhibitors to ERK, COX-2, or PKC (b, d) and proliferation assessed by incorporation of [3H]-thymidine as described in Materials and Methods. Data shown are mean ± SEM of four experiments conducted in triplicate. *, P < 0.01 compared with serum-free normal Fb; **, P < 0.01 compared with serum-free IPF-Fb.
Figure 9.
IL-11 inhibits expression of ERK-dependent p27Kip1 expression in normal Fb and IPF-Fb. Cells were incubated in serum-free media and exposed to IL-11 (50 ng/ml) in the presence or absence of PD98059. Lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and the expression of p27Kip1, cyclin D1, cyclin E1, and phosphorylated retinoblastoma protein assessed by Western blot analysis as described in Materials and Methods. The figure is representative of three separate experiments.
Similar articles
- Interleukin-6 and oncostatin M-induced growth inhibition of human A375 melanoma cells is STAT-dependent and involves upregulation of the cyclin-dependent kinase inhibitor p27/Kip1.
Kortylewski M, Heinrich PC, Mackiewicz A, Schniertshauer U, Klingmüller U, Nakajima K, Hirano T, Horn F, Behrmann I. Kortylewski M, et al. Oncogene. 1999 Jun 24;18(25):3742-53. doi: 10.1038/sj.onc.1202708. Oncogene. 1999. PMID: 10391682 - Soluble mannose 6-phosphate/insulin-like growth factor II (IGF-II) receptor inhibits interleukin-6-type cytokine-dependent proliferation by neutralization of IGF-II.
Duplomb L, Chaigne-Delalande B, Vusio P, Raher S, Jacques Y, Godard A, Blanchard F. Duplomb L, et al. Endocrinology. 2003 Dec;144(12):5381-9. doi: 10.1210/en.2003-0607. Epub 2003 Aug 28. Endocrinology. 2003. PMID: 12959977 - Effects of thrombopoietin, interleukin-3 and the kinase inhibitor K-252a on growth and polyploidization of the megakaryocytic cell line M-07e.
Quentmeier H, Zaborski M, Drexler HG. Quentmeier H, et al. Leukemia. 1998 Oct;12(10):1603-11. doi: 10.1038/sj.leu.2401170. Leukemia. 1998. PMID: 9766506 - Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway.
Heinrich PC, Behrmann I, Müller-Newen G, Schaper F, Graeve L. Heinrich PC, et al. Biochem J. 1998 Sep 1;334 ( Pt 2)(Pt 2):297-314. doi: 10.1042/bj3340297. Biochem J. 1998. PMID: 9716487 Free PMC article. Review. - Role of cyclin-dependent kinases and their inhibitors in cellular differentiation and development.
Chellappan SP, Giordano A, Fisher PB. Chellappan SP, et al. Curr Top Microbiol Immunol. 1998;227:57-103. doi: 10.1007/978-3-642-71941-7_4. Curr Top Microbiol Immunol. 1998. PMID: 9479826 Review. No abstract available.
Cited by
- Emerging pharmacological options in the treatment of idiopathic pulmonary fibrosis (IPF).
Aribindi K, Liu GY, Albertson TE. Aribindi K, et al. Expert Rev Clin Pharmacol. 2024 Sep;17(9):817-835. doi: 10.1080/17512433.2024.2396121. Epub 2024 Aug 27. Expert Rev Clin Pharmacol. 2024. PMID: 39192604 Review. - The Role of Cytokines and Molecular Pathways in Lung Fibrosis Following SARS-CoV-2 Infection: A Physiopathologic (Re)view.
Lazar M, Sandulescu M, Barbu EC, Chitu-Tisu CE, Andreescu DI, Anton AN, Erculescu TM, Petre AM, Duca GT, Simion V, Padiu IF, Pacurar CG, Rosca R, Simian TM, Oprea CA, Ion DA. Lazar M, et al. Biomedicines. 2024 Mar 13;12(3):639. doi: 10.3390/biomedicines12030639. Biomedicines. 2024. PMID: 38540252 Free PMC article. Review. - Prevention of bleomycin-induced pulmonary fibrosis by vaccination with the Tocilizumab mimotope.
Guo J, Yang L, Song H, Bai L. Guo J, et al. Hum Vaccin Immunother. 2024 Dec 31;20(1):2319965. doi: 10.1080/21645515.2024.2319965. Epub 2024 Feb 26. Hum Vaccin Immunother. 2024. PMID: 38408907 Free PMC article. - Perspectives on Post-COVID-19 Pulmonary Fibrosis Treatment.
Cojocaru E, Cojocaru T, Pînzariu GM, Vasiliu I, Armașu I, Cojocaru C. Cojocaru E, et al. J Pers Med. 2023 Dec 29;14(1):51. doi: 10.3390/jpm14010051. J Pers Med. 2023. PMID: 38248752 Free PMC article. Review. - Association between COVID-19 and sexual health: an umbrella review.
Liu WY, Jiesisibieke ZL, Chien CW, Tung TH. Liu WY, et al. Ann Med. 2023;55(2):2258902. doi: 10.1080/07853890.2023.2258902. Epub 2023 Sep 21. Ann Med. 2023. PMID: 37733015 Free PMC article.
References
- Bjoraker JA, Ryu JH, Edwin MK, Myers JL, Tazelaar HD, Schroeder DR, Offord KP: Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1998, 157:199-203 - PubMed
- Selman M, King TE, Pardo A: Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 2001, 134:136-151 - PubMed
- Mason RJ, Schwarz MI, Hunninghake GW, Musson RA: NHLBI Workshop Summary. Pharmacological therapy for idiopathic pulmonary fibrosis. Past, present, and future. Am J Respir Crit Care Med 1999, 160:1771-1777 - PubMed
- Gauldie J: Inflammatory mechanisms are a minor component of the pathogenesis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2002, 165:1205-1206 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous