The role of cGMP in the regulation of rabbit airway ciliary beat frequency - PubMed (original) (raw)
The role of cGMP in the regulation of rabbit airway ciliary beat frequency
Luo Zhang et al. J Physiol. 2003.
Abstract
The involvement of cyclic guanosine 3',5'-monophosphate (cGMP) and cGMP-dependent protein kinase (PKG) and their interaction with the Ca2+-dependent mechanisms in the regulation of ciliary activity are not well understood. To investigate how cGMP regulates ciliary activity, changes in ciliary beat frequency (CBF) and intracellular calcium concentration ([Ca2+]i) of rabbit tracheal ciliated cells in response to 8-bromo-cGMP (Br-cGMP) were simultaneously quantified using digital, high-speed phase-contrast and fluorescence imaging. Br-cGMP induced a response in ciliary activity that could be separated into two parts. Firstly, Br-cGMP induced a concentration-dependent increase in the basal CBF that occurred without increasing the [Ca2+]i. This response was not affected by excessively buffering the [Ca2+]i with BAPTA but was abolished by KT5823, a PKG inhibitor. Secondly, Br-cGMP induced a series of transient increases in CBF that were superimposed on the sustained increases in CBF. These transient increases in CBF correlated with the stimulation of a series of transient increases in [Ca2+]i and were abolished by BAPTA, but were unaffected by KT5823. The magnitude of the transient increases in CBF and [Ca2+]i were not dependent on the concentration of Br-cGMP. The Ca2+-dependent changes in CBF induced by ionomycin or ATP were not affected by KT5823. From these results, we propose that cGMP increases CBF in two ways: firstly through a Ca2+-independent mechanism involving PKG, and secondly through a Ca2+-dependent mechanism following the stimulation of changes in [Ca2+]i. In addition, we suggest that the Ca2+-dependent stimulation of rabbit airway ciliary activity does not initially require PKG activation.
Figures
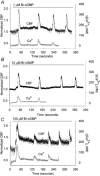
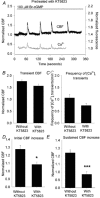
Figure 1. The effects of 8-Bromo-cGMP (Br-cGMP) on the ciliary beat frequency (CBF) and [Ca2+]i of rabbit airway ciliated cells
Data for both CBF and [Ca2+]i are normalized with the initial basal value and are representative of multiple cells. A, 1 μM Br-cGMP induced a two-part response in the CBF consisting of a small increase in the basal CBF (basal CBF = 10.4 Hz) that reached a maximum level within 60 s and declined thereafter, and larger transient increases in CBF that were superimposed on the basal CBF. By contrast, Br-cGMP only induced a series of transient increases in the [Ca2+]i without affecting the basal [Ca2+]i (n = 10). B, 10 μM Br-cGMP induced a higher sustained increase in the basal CBF (basal CBF = 15.9 Hz) but the superimposed transient increases in CBF, as well as the transient increases in [Ca2+]i, were unchanged (n = 11). C, 100 μM Br-cGMP only induced a further increase in the sustained CBF increase (basal CBF = 14.1 Hz) (n = 11). A-C, in all cases, the sustained increases in CBF occurred in the absence of changes in [Ca2+]i while the transient changes in CBF and [Ca2+]i were tightly coupled.
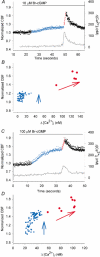
Figure 2. The correlation of the initial changes in CBF (coloured dots) with changes in [Ca2+]i (grey line) induced by 10 (A and B) or 100 μM (C and D) Br-cGMP
A, after the addition of 10 μM Br-cGMP, the CBF (normalized with basal CBF) slowly increased (blue dots) from the basal CBF (17.9 Hz, left black dots) while the [Ca2+]i remained unchanged. Approximately 30 s after the addition of Br-cGMP, the first transient increase in [Ca2+]i occurred and this was strongly correlated with a transient increase in CBF (red dots). B, the normalized CBF plotted against the corresponding [Ca2+]i for the time period illustrated in A. The vertical distribution of the blue dots, which represent the initial CBF increase induced by Br-cGMP, indicate that this phase of the response occurs independently of the [Ca2+]i. The inclined distribution of the red dots, which represent the CBF during the transient increase in [Ca2+]i, indicate that the CBF is dependent on the [Ca2+]i during this phase of the response. C and D, similar temporal and correlative representations of the changes in CBF and [Ca2+]i induced by 100 μM Br-cGMP. The initial increase in CBF (blue dots) is larger (basal CBF = 14.8 Hz) but still independent of the [Ca2+]i. The transient changes in CBF (red dots) are dependent on the [Ca2+]i.
Figure 3. The concentration-response relationship of CBF and [Ca2+]i in airway epithelial cells to Br-cGMP
A, the initial increase in CBF (○) in response to 1, 10 and 100 μM Br-cGMP was 1.13 ± 0.02 (n = 10), 1.23 ± 0.04 (n = 11) and 1.28 ± 0.05, (n = 11), respectively, and was significantly greater (all P < 0.001) with respect to the basal rate with the increasing Br-cGMP concentration. The CBF increase induced by 100 μM Br-cGMP was also significantly greater than that induced by 1 μM Br-cGMP (*_P_ < 0.05). The sustained increase in CBF (□) in response to 1, 10 and 100 μM Br-cGMP was 1.02 ± 0.02 (_n_ = 10), 1.17 ± 0.04 (_n_ = 11) and 1.24 ± 0.04 (_n_ = 11), respectively, and this was also significantly greater (all _P_ < 0.001) with the increasing Br-cGMP concentration. The sustained increase in CBF induced by 10 and 100 μM Br-cGMP was also significantly greater (**_P_ < 0.01, ***_P_ < 0.001) than that induced by 1 μM Br-cGMP. The transient increases in CBF (▵) in response to 1, 10 and 100 μM Br-cGMP were 1.60 ± 0.08 (_n_ = 10), 1.73 ± 0.08 (_n_ = 10) and 1.77 ± 0.06 (_n_ = 11), respectively, but these were not significantly increased with increasing Br-cGMP concentration. The starting basal CBF (12.8 ± 0.9 Hz, _n_ = 10; 11.9 ± 0.9 Hz, _n_ = 11; 12.4 ± 0.6 Hz, _n_ = 11) for each concentration of Br-cGMP was similar (_P_ > 0.05). B, the concentration-response relationship of the transient change in [Ca2+]i to Br-cGMP. The transient changes in [Ca2+]i (♦) in response to 1, 10 and 100 μM Br-cGMP were 83 ± 10 nM, (n = 10), 94 ± 10 nM (n = 9) and 92 ± 10 nM (n = 10), respectively, but were not significantly different with increasing Br-cGMP concentration (P > 0.05). The basal [Ca2+]i (30 ± 4 nM, n = 10; 36 ± 4 nM, n = 11; 32 ± 5 nM, n = 11) for each concentration was similar (P > 0.05). C, the concentration-response relationship of the frequency of the transient changes in [Ca2+]i or CBF oscillations (•) to Br-cGMP. The frequency in response to 1, 10 and 100 μM Br-cGMP was 0.67 ± 0.02 min−1 (n = 5), 0.82 ± 0.13 min−1 (n = 5) and 1.07 ± 0.14 min−1 (n = 4), and not significantly different with the increasing Br-cGMP concentration (P > 0.05).
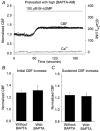
Figure 4. The effect of buffering the [Ca2+]i with BAPTA in response to 100 μM Br-cGMP
A, a representative response of a ciliated cell pretreated with 20 μM BAPTA AM and exposed to Br-cGMP (bar). The BAPTA buffering prevented any changes in [Ca2+]i (grey line). However, the CBF (black dots) still increased (to an initial normalized CBF = 1.36) and remained sustained (at a normalized CBF = 1.32). Transient increases in [Ca2+]i or CBF did not occur. The basal CBF was 12.1 Hz. B and C, the effect of buffering the [Ca2+]i with BAPTA on the initial (B) and sustained (C) changes in CBF induced by Br-cGMP. In control experiments, without BAPTA treatment, the initial and sustained increases in CBF induced by Br-cGMP were 1.28 ± 0.05 (n = 11) and 1.24 ± 0.04 (n = 11) respectively. These changes were indistinguishable (P > 0.05) from the initial (1.32 ± 0.08, n = 6) and sustained (1.23 ± 0.05, n = 6) increases in CBF induced by Br-cGMP in the presence of BAPTA. The basal CBFs for cells with (12.4 ± 1.0, n = 6) or without BAPTA treatment (12.4 ± 0.6, n = 11) were similar (P > 0.05).
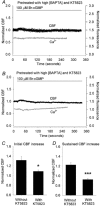
Figure 5. The combined effect of buffering [Ca2+]i with BAPTA and inhibiting PKG activity with KT5823 on the response to Br-cGMP
A and B, two representative cells illustrate the combined effect of the pretreatment of cells with KT5823 and BAPTA AM on the changes in CBF (black dots) and [Ca2+]i (grey lines) induced by 100 μM Br-cGMP (bar). KT5823 and BAPTA AM treatment prevented any significant increases in the [Ca2+]i and CBF in response to Br-cGMP. The basal CBF of the cells was 17.7 Hz (A) and 12.5 Hz (B). C and D, summaries of the combined effects of KT5823 and BAPTA AM on the initial (C) and sustained (D) increases in CBF induced by 100 μM Br-cGMP. The increase in the initial CBF (1.09 ± 0.03, n = 7) induced by Br-cGMP in the presence of KT5823 and BAPTA AM was significantly lower than the increase induced in cells without treatment (1.32 ± 0.08, n = 6, *P < 0.05). Similarly, the sustained increase in CBF (0.93 ± 0.03, _n_ = 7) was significantly lower compared to cells without treatment (1.23 ± 0.05, _n_ = 6, ***_P_ < 0.001). The basal CBFs from each group were similar (12.4 ± 1.0 Hz, _n_ = 6 and 15.3 ± 1.2 Hz, _n_ = 7, _P_ > 0.05).
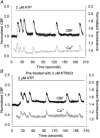
Figure 6. The effect of KT5823 treatment on changes in CBF and [Ca2+]i induced by 100 μM Br-cGMP
A, Br-cGMP (bar) induced a very small initial increase in CBF (black dots) that rapidly returned to the basal rate without inducing significant increases in [Ca2+]i (grey line). However, Br-cGMP still induced a series of transients in [Ca2+]i accompanied with transient increases in CBF. The basal CBF was 15.2 Hz. B and C, summaries of the effects of KT5823 treatment on Br-cGMP-induced transient changes in CBF (B) and the frequency of [Ca2+]i transients (C). KT5823 had no significant effect on the transient CBF (1.56 ± 0.08, n = 6 versus 1.77 ± 0.06, n = 11, P > 0.05) or the frequency of [Ca2+]i transients (0.74 ± 0.09, n = 4 versus 1.07 ± 0.14, n = 4, P > 0.05) as compared to controls. The basal CBFs from each group were similar (14.1 ± 0.9 Hz, n = 6 and 12.4 ± 0.6 Hz, n = 11, P > 0.05). D and E, summaries of the effects of KT5823 on initial (D) and sustained (E) CBF induced by Br-cGMP. Br-cGMP at 100 μM induced a significantly lower initial increase in CBF (1.08 ± 0.03, n = 6) compared to cells without KT5823 treatment (1.28 ± 0.05, n = 11, P < 0.05) and a significantly lower sustained CBF increase (0.97 ± 0.04, n = 6) compared to cells without KT5823 treatment (1.24 ± 0.04, n = 11, P < 0.001). *P < 0.05, ***P < 0.001.
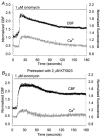
Figure 7. The effect of KT5823 treatment on ionomycin-induced increases in CBF and [Ca2+]i
A, a representative trace (n = 5) of the simultaneous changes in CBF (black dots) and [Ca2+]i (grey line) in a ciliated epithelial cell in response to 1 μM ionomycin (bar). Ionomycin induced a rapid increase in CBF and [Ca2+]i. While the [Ca2+]i gradually returned to the basal level, the CBF remained sustained at an elevated level. B, following the pretreatment of cells with 2 μM KT5823, the response induced by 1 μM ionomycin was indistinguishable from the control response (n = 5).
Figure 8. The effect of KT5823 treatment on ATP-induced changes in CBF and [Ca2+]i
A, a representative trace of the simultaneous changes in CBF (black dots) and [Ca2+]i (grey line) in ciliated epithelial cells in response to 2 μM ATP (bar). ATP induced a rapid increases in CBF and [Ca2+]i which was followed by oscillations in both CBF and [Ca2+]i. The CBF oscillations occurred from an elevated minimum CBF while the [Ca2+]i oscillations occurred from a baseline that declined to the basal level. B, ATP induced a similar response in CBF and [Ca2+]i in cells that were pretreated with 2 μM KT5823 (n = 5).
Similar articles
- Regulation of ciliary beat frequency by the nitric oxide-cyclic guanosine monophosphate signaling pathway in rat airway epithelial cells.
Li D, Shirakami G, Zhan X, Johns RA. Li D, et al. Am J Respir Cell Mol Biol. 2000 Aug;23(2):175-81. doi: 10.1165/ajrcmb.23.2.4022. Am J Respir Cell Mol Biol. 2000. PMID: 10919983 - Oscillations in ciliary beat frequency and intracellular calcium concentration in rabbit tracheal epithelial cells induced by ATP.
Zhang L, Sanderson MJ. Zhang L, et al. J Physiol. 2003 Feb 1;546(Pt 3):733-49. doi: 10.1113/jphysiol.2002.028704. J Physiol. 2003. PMID: 12563000 Free PMC article. - Effect of isoproterenol on the regulation of rabbit airway ciliary beat frequency measured with high-speed digital and fluorescence microscopy.
Zhang L, Han D, Sanderson MJ. Zhang L, et al. Ann Otol Rhinol Laryngol. 2005 May;114(5):399-403. doi: 10.1177/000348940511400512. Ann Otol Rhinol Laryngol. 2005. PMID: 15966529 - Regulation of mammalian ciliary beating.
Salathe M. Salathe M. Annu Rev Physiol. 2007;69:401-22. doi: 10.1146/annurev.physiol.69.040705.141253. Annu Rev Physiol. 2007. PMID: 16945069 Review. - Ciliary beat co-ordination by calcium.
Schmid A, Salathe M. Schmid A, et al. Biol Cell. 2011 Apr;103(4):159-69. doi: 10.1042/BC20100120. Biol Cell. 2011. PMID: 21401526 Review.
Cited by
- Apical oxidative hyaluronan degradation stimulates airway ciliary beating via RHAMM and RON.
Manzanares D, Monzon ME, Savani RC, Salathe M. Manzanares D, et al. Am J Respir Cell Mol Biol. 2007 Aug;37(2):160-8. doi: 10.1165/rcmb.2006-0413OC. Epub 2007 Mar 29. Am J Respir Cell Mol Biol. 2007. PMID: 17395888 Free PMC article. - In vitro culturing of porcine tracheal mucosa as an ideal model for investigating the influence of drugs on human respiratory mucosa.
Stennert E, Siefer O, Zheng M, Walger M, Mickenhagen A. Stennert E, et al. Eur Arch Otorhinolaryngol. 2008 Sep;265(9):1075-81. doi: 10.1007/s00405-008-0661-5. Epub 2008 May 6. Eur Arch Otorhinolaryngol. 2008. PMID: 18458926 Free PMC article. - Broncho-Vaxom® (OM-85 BV) soluble components stimulate sinonasal innate immunity.
Triantafillou V, Workman AD, Patel NN, Maina IW, Tong CCL, Kuan EC, Kennedy DW, Palmer JN, Adappa ND, Waizel-Haiat S, Cohen NA. Triantafillou V, et al. Int Forum Allergy Rhinol. 2019 Apr;9(4):370-377. doi: 10.1002/alr.22276. Epub 2019 Jan 7. Int Forum Allergy Rhinol. 2019. PMID: 30615298 Free PMC article. - Airway Epithelial Cell Cilia and Obstructive Lung Disease.
Yaghi A, Dolovich MB. Yaghi A, et al. Cells. 2016 Nov 11;5(4):40. doi: 10.3390/cells5040040. Cells. 2016. PMID: 27845721 Free PMC article. Review. - Methods to measure and analyze ciliary beat activity: Ca2+ influx-mediated cilia mechanosensitivity.
Li WE, Chen W, Ma YF, Tuo QR, Luo XJ, Zhang T, Sai WB, Liu J, Shen J, Liu ZG, Zheng YM, Wang YX, Ji G, Liu QH. Li WE, et al. Pflugers Arch. 2012 Dec;464(6):671-80. doi: 10.1007/s00424-012-1164-1. Epub 2012 Oct 5. Pflugers Arch. 2012. PMID: 23053477 Free PMC article.
References
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. - PubMed
- Bonini NM, Nelson DL. Phosphoproteins associated with cyclic nucleotide stimulation of ciliary motility in Paramecium. J Cell Sci. 1990;95:219–230. - PubMed
- Braiman A, Silberberg SD, Priel Z. Purinergic stimulation of ciliary activity. Drug Dev Res. 2000;50:550–554.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous