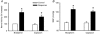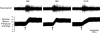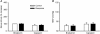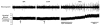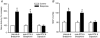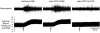Cardiac vanilloid receptor 1-expressing afferent nerves and their role in the cardiogenic sympathetic reflex in rats - PubMed (original) (raw)
Cardiac vanilloid receptor 1-expressing afferent nerves and their role in the cardiogenic sympathetic reflex in rats
Matthew R Zahner et al. J Physiol. 2003.
Abstract
Myocardial ischaemia causes the release of metabolites such as bradykinin, which stimulates cardiac sensory receptors to evoke a sympathoexcitatory reflex. However, the molecular identity of the afferent neurons and fibres mediating this reflex response is not clear. In this study, we tested the hypothesis that the cardiogenic sympathoexcitatory reflex is mediated by capsaicin-sensitive afferent fibres. Enhanced immunofluorescence labelling revealed that vanilloid receptor 1 (VR1)-containing afferent nerve fibres were present on the epicardial surface of the rat heart. Resiniferatoxin (RTX), a potent analogue of capsaicin, was used to deplete capsaicin-sensitive afferent fibres in rats. Depletion of these fibres was confirmed by a substantial reduction of VR1 immunoreactivity in the epicardium and dorsal root ganglia. The thermal sensitivity was also diminished in RTX-treated rats. Renal sympathetic nerve activity (RSNA) and blood pressure were recorded in anaesthetized rats during epicardial application of bradykinin or capsaicin. In vehicle-treated rats, epicardial bradykinin (10 microg ml-1) or capsaicin (10 microg ml-1) application produced a significant increase in RSNA and arterial blood pressure. The RSNA and blood pressure responses caused by bradykinin and capsaicin were completely abolished in RTX-treated rats. Furthermore, epicardial application of iodo-RTX, a highly specific antagonist of VR1 receptors, blocked capsaicin- but not bradykinin-induced sympathoexcitatory responses. Thus, these data provide important histological and functional evidence that the heart is innervated by VR1-expressing afferent nerves and these afferent nerves are essential for the cardiogenic sympathoexcitatory reflex during myocardial ischaemia.
Figures
Figure 1
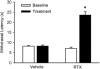
Mean paw withdrawal latency in response to a radiant heat stimulus before and 5 days after RTX (200 μg kg−1, n = 14) or vehicle (n = 18) treatment. Data are presented as means ±
s.e.m
. *P < 0.05 compared with baseline values.
Figure 2
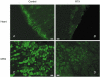
Confocal images showing immunofluorescence labelling of VR1 receptors in the left ventricle of the heart (scale bars: 15 μm in A and B) and thoracic dorsal root ganglia (DRG, scale bars: 30 μm in C and D) in a vehicle-treated (Control) and an RTX-treated rat. Note the dense VR1 immunofluorescence on the epicardial surface of the heart in the control rat.
Figure 4
Group data showing the RSNA (A) and mean arterial pressure (MAP, B) responses to epicardial application of bradykinin (10 μg ml−1, n = 10) and capsaicin (10 μg ml−1, n = 9). RSNA responses evoked by bradykinin and capsaicin were measured as the percentage change from baseline RSNA prior to bradykinin application. Data are presented as means ±
s.e.m
. *P < 0.05 compared with respective controls.
Figure 3
Original tracings from a vehicle-treated rat showing RSNA and blood pressure responses to epicardial application of bradykinin (BK, 10 μg ml−1) and capsaicin (CAP, 10 μg ml−1). Bradykinin or capsaicin was applied at the time points indicated by the arrows.
Figure 6
Group data showing RSNA (A) and mean arterial pressure (MAP, B) responses to epicardial application of bradykinin (10 μg ml−1, n = 9) and capsaicin (10 μg ml−1, n = 9). RSNA responses evoked by bradykinin and capsaicin were measured as the percentage change from baseline RSNA prior to bradykinin application. Data are presented as means ±
s.e.m
.
Figure 5
Original tracings showing lack of RSNA and blood pressure responses to epicardial application of bradykinin (BK, 10 μg ml−1) and capsaicin (CAP, 10 μg ml−1) in an RTX-treated rat. Intracerebroventricular bicuculline (BIC, 10 μg in 10 μl vehicle) injection increased blood pressure and RSNA. Bradykinin or capsaicin was applied at the time points indicated by the arrows.
Figure 8
Group data showing RSNA (A) and mean arterial pressure (MAP, B) responses to epicardial bradykinin (10 μg ml−1) plus vehicle or iodo-RTX (200 μ
m
), and capsaicin (10 μg ml−1) plus iodo-RTX in 7 normal control rats. The RSNA following bradykinin or capsaicin application is presented as the percentage change from baseline RSNA. Data are presented as means ±
s.e.m
. *P < 0.05 compared with respective controls.
Figure 7
Original tracings showing the responses of RSNA and arterial blood pressure to epicardial application of vehicle, capsaicin (10 μg ml−1) and bradykinin (10 μg ml−1) in the presence of iodo-RTX (200 μ
m
) in a normal rat. Bradykinin or capsaicin was applied at the time points indicated by the arrows.
Comment in
- The spice of life is at the root of cardiac pain.
Schultz HD. Schultz HD. J Physiol. 2003 Sep 1;551(Pt 2):400. doi: 10.1113/jphysiol.2003.050104. Epub 2003 Jul 22. J Physiol. 2003. PMID: 12876213 Free PMC article. No abstract available.
Similar articles
- Sympatho-excitatory response to pulmonary chemosensitive spinal afferent activation in anesthetized, vagotomized rats.
Shanks J, Xia Z, Lisco SJ, Rozanski GJ, Schultz HD, Zucker IH, Wang HJ. Shanks J, et al. Physiol Rep. 2018 Jun;6(12):e13742. doi: 10.14814/phy2.13742. Physiol Rep. 2018. PMID: 29906340 Free PMC article. - Central AT1 receptors are involved in the enhanced cardiac sympathetic afferent reflex in rats with chronic heart failure.
Zhu GQ, Zucker IH, Wang W. Zhu GQ, et al. Basic Res Cardiol. 2002 Jul;97(4):320-6. doi: 10.1007/s00395-002-0353-z. Basic Res Cardiol. 2002. PMID: 12111042 - Cardiac vanilloid receptor-1 afferent depletion enhances stellate ganglion neuronal activity and efferent sympathetic response to cardiac stress.
Yoshie K, Rajendran PS, Massoud L, Kwon O, Tadimeti V, Salavatian S, Ardell JL, Shivkumar K, Ajijola OA. Yoshie K, et al. Am J Physiol Heart Circ Physiol. 2018 May 1;314(5):H954-H966. doi: 10.1152/ajpheart.00593.2017. Epub 2018 Jan 16. Am J Physiol Heart Circ Physiol. 2018. PMID: 29351450 Free PMC article. - Cardiac nociceptive reflexes: role of kinins, prostanoids and capsaicin-sensitive afferents.
Staszewska-Woolley J, Woolley G. Staszewska-Woolley J, et al. Pol J Pharmacol Pharm. 1990 May-Jun;42(3):237-47. Pol J Pharmacol Pharm. 1990. PMID: 2263534 Review. - Capsaicin sensitive-sensory nerves and blood pressure regulation.
Vaishnava P, Wang DH. Vaishnava P, et al. Curr Med Chem Cardiovasc Hematol Agents. 2003 Jun;1(2):177-88. doi: 10.2174/1568016033477540. Curr Med Chem Cardiovasc Hematol Agents. 2003. PMID: 15320697 Review.
Cited by
- Spinal cord astrocytes regulate myocardial ischemia-reperfusion injury.
Wu C, Liu R, Luo Z, Sun M, Qile M, Xu S, Jin S, Zhang L, Gross ER, Zhang Y, He S. Wu C, et al. Basic Res Cardiol. 2022 Nov 11;117(1):56. doi: 10.1007/s00395-022-00968-x. Basic Res Cardiol. 2022. PMID: 36367592 Free PMC article. - Investigating the involvement of TRPV1 ion channels in remote hind limb preconditioning-induced cardioprotection in rats.
Randhawa PK, Jaggi AS. Randhawa PK, et al. Naunyn Schmiedebergs Arch Pharmacol. 2017 Feb;390(2):117-126. doi: 10.1007/s00210-016-1311-x. Epub 2016 Oct 17. Naunyn Schmiedebergs Arch Pharmacol. 2017. PMID: 27752734 - Transient receptor potential vanilloid type 1: cardioprotective effects in diabetic models.
Bao J, Gao Z, Hu Y, Ye L, Wang L. Bao J, et al. Channels (Austin). 2023 Dec;17(1):2281743. doi: 10.1080/19336950.2023.2281743. Epub 2023 Nov 20. Channels (Austin). 2023. PMID: 37983306 Free PMC article. Review. - Endogenous endothelin stimulates cardiac sympathetic afferents during ischaemia.
Fu LW, Guo ZL, Longhurst JC. Fu LW, et al. J Physiol. 2010 Jul 1;588(Pt 13):2473-86. doi: 10.1113/jphysiol.2010.188730. Epub 2010 May 4. J Physiol. 2010. PMID: 20442267 Free PMC article. - Selective sensory denervation by capsaicin aggravates adriamycin-induced cardiomyopathy in rats.
Katona M, Boros K, Sántha P, Ferdinandy P, Dux M, Jancsó G. Katona M, et al. Naunyn Schmiedebergs Arch Pharmacol. 2004 Dec;370(6):436-43. doi: 10.1007/s00210-004-0985-7. Epub 2004 Nov 10. Naunyn Schmiedebergs Arch Pharmacol. 2004. PMID: 15549271
References
- Avelino A, Cruz C, Nagy I, Cruz F. Vanilloid receptor 1 expression in the rat urinary tract. Neuroscience. 2002;109:787–798. - PubMed
- Barber MJ, Mueller TM, Davies BG, Zipes DP. Phenol topically applied to canine left ventricular epicardium interrupts sympathetic but not vagal afferents. Circ Res. 1984;55:532–544. - PubMed
- Blair RW, Weber RN, Foreman RD. Responses of thoracic spinothalamic neurons to intracardiac injection of bradykinin in the monkey. Circ Res. 1982;51:83–94. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources