Structure-based mutagenesis of herpes simplex virus glycoprotein D defines three critical regions at the gD-HveA/HVEM binding interface - PubMed (original) (raw)
Structure-based mutagenesis of herpes simplex virus glycoprotein D defines three critical regions at the gD-HveA/HVEM binding interface
Sarah A Connolly et al. J Virol. 2003 Jul.
Abstract
Herpes simplex virus (HSV) entry into cells requires the binding of glycoprotein D (gD) to one of several cell surface receptors. The crystal structure of gD bound to one of these receptors, HveA/HVEM, reveals that the core of gD comprises an immunoglobulin fold flanked by a long C-terminal extension and an N-terminal hairpin loop. HveA is a member of the tumor necrosis factor receptor family and contains four cysteine-rich domains (CRDs) characteristic of this family. Fourteen amino acids within the gD N-terminal loop comprise the entire binding site for HveA. To determine the contribution of each gD contact residue to virus entry, we constructed gD molecules mutated in these amino acids. We determined the abilities of the gD mutants to bind receptors, facilitate virus entry, and mediate cell-cell fusion. Seven of the gD mutants exhibited wild-type levels of receptor binding and gD function. Results from the other seven gD mutants revealed three critical regions at the gD-HveA interface. (i) Several gD residues that participate in an intermolecular beta-sheet with HveA were found to be crucial for HveA binding and entry into HveA-expressing cells. (ii) Two gD residues that contact HveA-Y23 contributed to HveA binding but were not required for mediating entry into cells. HveA-Y23 fits into a crevice on the surface of gD and was previously shown to be essential for gD binding. (iii) CRD2 was previously shown to contribute to gD binding, and this study shows that one gD residue that contacts CRD2 contributes to HveA binding. None of the gD mutations prevented interaction with nectin-1, another gD receptor. However, when cotransfected with the other glycoproteins required for fusion, two gD mutants gained the ability to mediate fusion of cells expressing nectin-2, a gD receptor that interacts with several laboratory-derived gD mutants but not with wild-type gD. Thus, results from this panel of gD mutants as well as those of previous studies (A. Carfi, S. H. Willis, J. C. Whitbeck, C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and D. C. Wiley, Mol. Cell 8:169-179, 2001, and S. A. Connolly, D. J. Landsburg, A. Carfi, D. C. Wiley, R. J. Eisenberg, and G. H. Cohen, J. Virol. 76:10894-10904, 2002) provide a detailed picture of the gD-HveA interface and the contacts required for functional interaction. The results demonstrate that of the 35 gD and HveA contact residues that comprise the gD-HveA interface, only a handful are critical for complex formation.
Figures
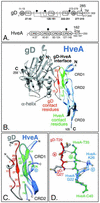
FIG. 1.
Highlighted regions within the gD-HveA complex. (A) Diagram of full-length gD and HveA. The amino acid numbers begin with the first residue in the mature protein after signal sequence cleavage. The positions of N glycosylation sites (black circles) and transmembrane regions (TM) are indicated. The gD amino acids comprising each of four defined functional regions (FR) and the group VII and IIb MAb epitopes (gray circles) are labeled. The disulfide bond pattern (dotted lines) and locations of cysteines (C) within gD are indicated. The HveA amino acids comprising each of the four CRDs are labeled. Arrows indicate the sites of truncation for the proteins used to solve the crystal structure. (B) Ribbon diagram of the crystal structure of gD bound to HveA. The N- and C-terminal residues observed in the crystal structure and the locations of HveA CRDs are indicated. The gD molecule is shown in gray, with the gD contact residues located within the gD N-terminal loop shownin red. An α-helix behind the loop is noted. The HveA molecule is shown in blue, with the HveA contact residues found within CRD1 and CRD2 shown in green. Contact residues were defined as amino acids containing atoms that come within 4 Å of the partner molecule (Table 1). Some of the contact residues are numbered for reference. (C) An enlarged view of the gD-HveA interface shown in the same orientation as that in panel B. gD contact residues are displayed in red, and HveA contact residues are displayed in green. Three β-strands are labeled (a, b, and c) for reference. (D) An intermolecular antiparallel β-sheet formed between gD and HveA. A short gD β-strand (residues 27 [gD-Q27] to 29 [gD-T29] in red, strand a) is hydrogen bonded (dotted lines) to HveA residues 35 (HveA-T35) to 37 within a β-strand of HveA (residues 35 to 40 [HveA-C40] in green, strand b). This gD-HveA β-sheet augments a two-stranded β-sheet formed between HveA residues 36 and 40 and a second HveA β-strand (residues 22 [HveA-G22] to 26 [HveA-K26] in blue, strand c). gD side chains are shown in gray, and HveA side chains are omitted for clarity. The three β-strands are shown in the same orientation as those in panel C and labeled (a, b, and c) for reference. Nitrogen (navy) and oxygen (yellow) atoms are indicated. These graphics were created by using Swiss-PdbViewer (23) and POV-Ray software.
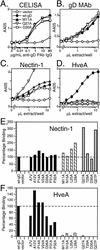
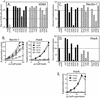
FIG. 2.
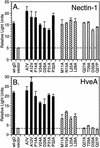
Expression and receptor binding of gD mutants. (A) Cell surface expression. B78-H1 cells transfected with plasmids encoding each of the gD mutants or wild-type gD (wt-gD) were seeded onto 96-well plates, and expression of gD on the cell surface was detected by using dilutions of anti-gD PAb. (B) gD quantitation by capture ELISA. Cell extracts were prepared from 293T cells transfected with plasmids encoding each of the gD mutants. Dilutions of extracts were added to 96-well plates coated with anti-gD MAb. The amount of gD captured by the MAb was detected by using anti-gD PAb. The cell extracts were then diluted in extraction buffer to obtain normalized levels of gD, and the capture ELISA was repeated for confirmation. Data are shown for normalized extracts of wild-type gD and three representative gD mutants. (C) Binding of gD mutants to nectin-1. Ninety-six-well plates were coated with a truncated form of nectin-1, incubated with dilutions of transfected cell extracts normalized for gD content, and probed with an anti-gD PAb to detect the levels of gD binding. Data are shown for wild-type gD and three gD mutants. (D) Binding of gD mutants to HveA. Ninety-six-well plates were coated with truncated forms of HveA and treated as described above. Assays detecting gD binding to HveA, nectin-1, and anti-gD MAb were run in parallel. (E) Nectin-1binding for all the gD mutants. Binding data for a single dilution of normalized extract (7.5 μl/well) are plotted. The negative control signal is subtracted, and receptor binding is expressed as a percentage of wild-type gD receptor binding. (F) HveA binding for all the gD mutants. Binding data for a single dilution of normalized extract (15 μl/well) are plotted as described above. The gD mutants are divided into three categories based on the overall phenotypes exhibited for interactions with HveA (Table 1). Category 1 mutants (black bars) have near wild-type levels of binding to both receptors. Category 2 (striped bars) and category 3 (gray bars) mutants have impaired binding to HveA but not nectin-1.
FIG. 3.
gD mutants mediating cell-cell fusion. CHO cells stably expressing either HveA (CHO-HVEM12) or nectin-1 (CHO-R3A) were transfected with a plasmid encoding the luciferase gene under the control of the T7 promoter and cocultivated with CHO-K1 cells transfected with plasmids encoding gB, gH, gL, a gD mutant, and T7 polymerase. The cells were lysed and assayed for luciferase activity as a measure of cell-cell fusion. The means and standard deviations of the results from one experiment performed in triplicate are shown. The gD mutants are divided into three categories (Table 1). Category 1 gD mutants (black bars) mediate near-wild-type levels of fusion of cells expressing either receptor. Category 2 mutants (striped bars) show reduced fusion of cells expressing HveA but wild-type levels of fusion of nectin-1-expressing cells. Category 3 mutants (gray bars) fail to mediate fusion of cells expressing HveA but mediate wild-type levels of fusion of nectin-1-expressing cells. wt-gD, wild-type gD.
FIG. 4.
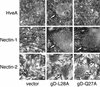
gD mutants mediating syncytium formation. B78-H1 cells stably expressing either HveA, nectin-1, or nectin-2 were transfected with plasmids encoding gB, gH, gL, and a gD mutant. Cells were stained and syncytia (arrows) were viewed by microscopy. The entire panel of gD mutants was tested. Sample data for two gD mutants are shown.
FIG. 5.
gD mutants mediating HSV entry into cells. L cells were transfected with plasmids encoding gD mutants, incubated overnight, and infected with gD-null HSV that had been phenotypically complemented with wild-type gD to allow for entry. Cell lysates containing progeny virions complemented with the gD mutants were harvested and assayed for virus entry activity. (A) Entry into VD60 cells. Dilutions of the complemented cell lysates were added to VD60 monolayers. After 2 days, cells were stained and plaques were counted. Titers are expressed as numbers of PFU per milliliter. wtgD, wild-type gD. (B) Entry into cells expressing nectin-1 and HveA. CHO cells that express either receptor and carry lacZ under control of the ICP4 promoter were incubated overnight with dilutions of complemented cell lysates. β-Galactosidase production was used as a measure of virus entry. Data are shown for wild-type gD and three gD mutants. (C and D) Entry mediated by the panel of gD mutants. The entry assay described for panel B was repeated for all gD mutants. For comparison, the β-galactosidase activities from a single dilution of the complemented cell lysates (50 μl/well) are shown. For each mutant, a negative control signal was subtracted and entry was expressed as a percentage of the entry mediated by wild-type gD. Category 1 (black bars) and category 2 (striped bars) mutants mediate entry into both nectin-1- and HveA-expressing cells. Category 3 mutants (gray bars) mediate entry into cells expressing nectin-1 but not those expressing HveA. (E) Entry of HSV complemented with gD-D26A into cells expressing HveA. Complemented virus was harvested from cell supernatants and assayed as described above. To compare the mutants, we plotted the β-galactosidase activity of each complemented virus lysate (at 50 μl/well) as a percentage of the β-galactosidase activity obtained when HSV was complemented with wild-type gD.
FIG. 6.
(A) Space-filling model of HveA and gD. The gD-HveA structure has been pried apart to expose the binding face of each protein. Residues that are critical for binding (red) or that contribute to binding (yellow) are indicated. HveA data are taken from reference . HveA-Y23 (red) fits into a crevice on the surface of gD (blue arrows). (B) The HveA binding site on gD. The gD-HveA interface from Fig. 1C is rotated so that the face of HveA binding on the gD N-terminal loop is shown. All gD contact residues are numbered, and the N and C termini are labeled. The space-filling spheres represent atoms of gD contact residues from category 1 (gray), category 2 (yellow), and category 3 (red) (Table 1). (C) The gD binding site on HveA. The gD-HveA interface from Fig. 1C is rotated so that the N-terminal loop of gD (black) lies on top of the HveA binding surface. HveA is viewed from the perspective of the α-helix (Fig. 1B). All HveA contact residues are numbered and shown in space-filling format. Individually mutating these residues results in wild-type or enhanced gD binding (gray), reduced gD binding (yellow), or a loss of gD binding (red) (12). The N and C termini of gD are labeled.
FIG. 7.
(A) Three critical regions at the gD-HveA interface. The interface from Fig. 1C is shown in two orientations. At the top of the interface, an intermolecular β-sheet (red) is formed between gD residues 27 to 29 and HveA residues 35 to 37. This β-sheet is critical for receptor binding and HSV entry. At the center of the interface, shown in green, HveA-Y23 contacts two gD residues on either side of the loop, gD-M11 and gD-L25, via a water molecule (w). HveA-Y23 is required for gD binding and HSV entry, while gD-M11 and gD-L25 contribute to HveA binding. Below this region, shown in yellow, gD-N15 contacts HveA CRD2 residues HveA-S74 and HveA-T76. These three residues contribute to complex formation. Dashed lines represent intermolecular hydrogen bonds. Oxygen (red), nitrogen (navy), and sulfur (white) atoms are indicated. (B) Location of four category 1 gD residues (red) and HveA residues (green). A salt bridge between gD-D26 and HveA-K26 (dotted lines) is indicated, as are nitrogen (navy) and oxygen (yellow) atoms. Main chain oxygen atoms are omitted for clarity.
Similar articles
- Structure-based analysis of the herpes simplex virus glycoprotein D binding site present on herpesvirus entry mediator HveA (HVEM).
Connolly SA, Landsburg DJ, Carfi A, Wiley DC, Eisenberg RJ, Cohen GH. Connolly SA, et al. J Virol. 2002 Nov;76(21):10894-904. doi: 10.1128/jvi.76.21.10894-10904.2002. J Virol. 2002. PMID: 12368332 Free PMC article. - Localization of the gD-binding region of the human herpes simplex virus receptor, HveA.
Whitbeck JC, Connolly SA, Willis SH, Hou W, Krummenacher C, Ponce de Leon M, Lou H, Baribaud I, Eisenberg RJ, Cohen GH. Whitbeck JC, et al. J Virol. 2001 Jan;75(1):171-80. doi: 10.1128/JVI.75.1.171-180.2001. J Virol. 2001. PMID: 11119586 Free PMC article. - Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry.
Krummenacher C, Nicola AV, Whitbeck JC, Lou H, Hou W, Lambris JD, Geraghty RJ, Spear PG, Cohen GH, Eisenberg RJ. Krummenacher C, et al. J Virol. 1998 Sep;72(9):7064-74. doi: 10.1128/JVI.72.9.7064-7074.1998. J Virol. 1998. PMID: 9696799 Free PMC article. - The role of herpes simplex virus glycoproteins in the virus replication cycle.
Rajcáni J, Vojvodová A. Rajcáni J, et al. Acta Virol. 1998 Apr;42(2):103-18. Acta Virol. 1998. PMID: 9770079 Review. - Different receptors binding to distinct interfaces on herpes simplex virus gD can trigger events leading to cell fusion and viral entry.
Spear PG, Manoj S, Yoon M, Jogger CR, Zago A, Myscofski D. Spear PG, et al. Virology. 2006 Jan 5;344(1):17-24. doi: 10.1016/j.virol.2005.09.016. Virology. 2006. PMID: 16364731 Review.
Cited by
- The amino terminus of varicella-zoster virus (VZV) glycoprotein E is required for binding to insulin-degrading enzyme, a VZV receptor.
Li Q, Krogmann T, Ali MA, Tang WJ, Cohen JI. Li Q, et al. J Virol. 2007 Aug;81(16):8525-32. doi: 10.1128/JVI.00286-07. Epub 2007 Jun 6. J Virol. 2007. PMID: 17553876 Free PMC article. - Nectin-2-mediated entry of a syncytial strain of herpes simplex virus via pH-independent fusion with the plasma membrane of Chinese hamster ovary cells.
Delboy MG, Patterson JL, Hollander AM, Nicola AV. Delboy MG, et al. Virol J. 2006 Dec 27;3:105. doi: 10.1186/1743-422X-3-105. Virol J. 2006. PMID: 17192179 Free PMC article. - A Herpes Simplex Virus 2 (HSV-2) gD Mutant Impaired for Neural Tropism Is Superior to an HSV-2 gD Subunit Vaccine To Protect Animals from Challenge with HSV-2.
Wang K, Goodman KN, Li DY, Raffeld M, Chavez M, Cohen JI. Wang K, et al. J Virol. 2015 Nov 11;90(1):562-74. doi: 10.1128/JVI.01845-15. Print 2016 Jan 1. J Virol. 2015. PMID: 26559846 Free PMC article. - The herpevac trial for women: Sequence analysis of glycoproteins from viruses obtained from infected subjects.
Minaya MA, Korom M, Wang H, Belshe RB, Morrison LA. Minaya MA, et al. PLoS One. 2017 Apr 27;12(4):e0176687. doi: 10.1371/journal.pone.0176687. eCollection 2017. PLoS One. 2017. PMID: 28448558 Free PMC article. - Herpesvirus Entry Mediator and Ocular Herpesvirus Infection: More than Meets the Eye.
Edwards RG, Longnecker R. Edwards RG, et al. J Virol. 2017 Jun 9;91(13):e00115-17. doi: 10.1128/JVI.00115-17. Print 2017 Jul 1. J Virol. 2017. PMID: 28404853 Free PMC article. Review.
References
- Bogan, A. A., and K. S. Thorn. 1998. Anatomy of hot spots in protein interfaces. J. Mol. Biol. 280:1-9. - PubMed
- Browne, H., B. Bruun, and T. Minson. 2001. Plasma membrane requirements for cell fusion induced by herpes simplex virus type 1 glycoproteins gB, gD, gH and gL. J. Gen. Virol. 82:1419-1422. - PubMed
- Campadelli-Fiume, G., F. Cocchi, L. Menotti, and M. Lopez. 2000. The novel receptors that mediate the entry of herpes simplex viruses and animal alphaherpesviruses into cells. Rev. Med. Virol. 10:305-319. - PubMed
- Carfi, A., H. Gong, H. Lou, S. H. Willis, G. H. Cohen, R. J. Eisenberg, and D. C. Wiley. 2002. Crystallization and preliminary diffraction studies of the ectodomain of the envelope glycoprotein D from herpes simplex virus 1 alone and in complex with the ectodomain of the human receptor HveA. Acta Crystallogr. Sect. D 58:836-838. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 NS030606/NS/NINDS NIH HHS/United States
- T32 AI007325/AI/NIAID NIH HHS/United States
- AI 18289/AI/NIAID NIH HHS/United States
- R01 NS036731/NS/NINDS NIH HHS/United States
- R01 AI018289/AI/NIAID NIH HHS/United States
- R37 AI018289/AI/NIAID NIH HHS/United States
- AI 07325/AI/NIAID NIH HHS/United States
- NS 30606/NS/NINDS NIH HHS/United States
- NS 36731/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous