Slx1-Slx4 is a second structure-specific endonuclease functionally redundant with Sgs1-Top3 - PubMed (original) (raw)
. 2003 Jul 15;17(14):1768-78.
doi: 10.1101/gad.1105203. Epub 2003 Jun 27.
Affiliations
- PMID: 12832395
- PMCID: PMC196184
- DOI: 10.1101/gad.1105203
Slx1-Slx4 is a second structure-specific endonuclease functionally redundant with Sgs1-Top3
William M Fricke et al. Genes Dev. 2003.
Abstract
The RecQ DNA helicases human BLM and yeast Sgs1 interact with DNA topoisomerase III and are thought to act on stalled replication forks to maintain genome stability. To gain insight into this mechanism, we previously identified SLX1 and SLX4 as genes that are required for viability and for completion of rDNA replication in the absence of SGS1-TOP3. Here we show that SLX1 and SLX4 encode a heteromeric structure-specific endonuclease. The Slx1-Slx4 nuclease is active on branched DNA substrates, particularly simple-Y, 5'-flap, or replication fork structures. It cleaves the strand bearing the 5' nonhomologous arm at the branch junction and generates ligatable nicked products from 5'-flap or replication fork substrates. Slx1 is the founding member of a family of proteins with a predicted URI nuclease domain and PHD-type zinc finger. This subunit displays weak structure-specific endonuclease activity on its own, is stimulated 500-fold by Slx4, and requires the PHD finger for activity in vitro and in vivo. Both subunits are required in vivo for resistance to DNA damage by methylmethane sulfonate (MMS). We propose that Sgs1-Top3 acts at the termination of rDNA replication to decatenate stalled forks, and, in its absence, Slx1-Slx4 cleaves these stalled forks.
Figures
Figure 1.
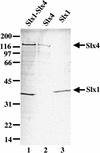
Purification of the Slx1–Slx4 complex. Approximately 1.9 μg of Slx1–Slx4, 1.4 μg of Slx4, and 0.5 μg of Slx1 were resolved by 10% SDS-PAGE as indicated, and detected by Coomassie blue staining. The Slx1 (36 kD) and Slx4 (130 kD) subunits are indicated. The ladder of low-intensity bands in lanes 1 and 2 is breakdown products of Slx4. Molecular mass standards (in kilodaltons) are indicated on the_left_.
Figure 2.
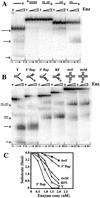
Substrate preferences of the Slx1–4 nuclease. Under standard assay conditions, 4 fmole of the indicated 5′-[32P]-labeled substrate was incubated with 0, 10, 20, 30, or 40 fmole of Slx1–4. The reactions were terminated, and the products were resolved by native 10% PAGE in 1× TBE. (A) Unbranched substrates were assembled from the following oligonucleotides: ssDNA (lanes 1–5), *891; dsDNA (lanes 6–10), *888/896; nicked DNA (lanes_11–15_), *891/994/1084; 3′ extension (lanes_16–20_), *891/994; and 5′ extension (lanes_16–20_), *891/1084. (B) Branched substrates were assembled from the following oligonucleotides: Y (lanes 1–5), 888/*891; 5′ flap (lanes 6–10), 888/*891/992; 3′ flap (lanes 11–15), 888/*891/994; replication fork (lanes_16–20_), 888/*891/992/994; fixed HJ (4wF, lanes_21–25_), 888/889/890/*891; and mobile HJ (4wM, lanes_26–30_), *892/893/894/895. (C) The quantity of undigested substrate remaining in the reactions shown in B was determined and is presented as a function of Slx1–4 concentration. The positions of reaction products are indicated on the left of_A_ and B. An asterisk represents the 5′-[32P] label.
Figure 3.
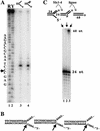
Holliday junction cleavage by Slx1–4. Four substrates were prepared by individually labeling each strand (892, 893, 894, or 895) of a HJ containing a 12-bp migratable core, and incubated with Slx1–4 under standard conditions (lanes 1_–_4). The digestion products, or a control substrate consisting of nicked duplex DNA (lane_7_), were then treated with DNA ligase (lanes_8_–12). All samples were subsequently resolved by sequencing gel electrophoresis. A chemical sequencing ladder of oligonucleotide 895 was used as a reference to identify sites of cleavage (lanes 5,6). (B) A schematic diagram of the central region of the HJ is presented summarizing the cleavage results obtained in_A_. The major (larger arrows) and minor (smaller arrows) sites of cleavage are indicated. The branch-migratable core is shown in gray. R, G + A; Y, T + C.
Figure 4.
5′-flap cleavage by Slx1–4. (A) The indicated Y and 5′-flap substrates were incubated with Slx1–4, and the reaction products were resolved by sequencing gel electrophoresis (lanes 3,4). Comparison to a chemical sequencing ladder of 5′-[32P]-labeled oligonucleotide 891 (lanes 1,2) revealed that hydrolysis occurred mainly at a single site (5′-GAGC↓CCTA-3′) on both substrates. Substrates were assembled as follows: Y, 888/*891; 5′ flap, 888/*891/992. R, G + A; Y, T + C. (B) Schematic diagrams indicate the sites of cleavage on three 5′-flap substrates of varying sequence adjacent to the branch site. (C) The indicated 5′-flap substrate (888/891/*992) was prepared in which the 24-nt downstream oligonucleotide was 32P-end-labeled (lane 1). The substrate was either incubated with Slx1–4 (lane_2_) or incubated with Slx1–4 followed by DNA ligase (lane_3_). Products were analyzed by sequencing gel electrophoresis.
Figure 5.
Slx1 and Slx4 subunits are required in vitro and in vivo. (A, left) The Y substrate was incubated with 0, 10, 100, or 1000 fmole of Slx1 (Slx1 lanes), Slx4 (Slx4 lanes), Slx1 and Slx4 that had been preincubated on ice for 30 min (Slx1 + Slx4), or the copurified Slx1–4 complex (Complex). The reaction products were then analyzed by native gel electrophoresis. Cleavage products are indicated by a lowercase letter at the_right_. (Right) Schematic diagram illustrating the sites of cleavage giving rise to the indicated products. (B) Strains of the indicated genotype were spotted in 10-fold serial dilutions on YPD plates or YPD plates containing the indicated concentrations of MMS. The plates were photographed following 3 d of growth at 30°C.
Figure 6.
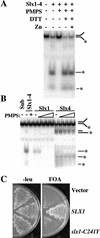
Cysteine residues of Slx1 are required in vitro and in vivo. (A) The Y substrate was incubated with 100 fmole of Slx1–4 that had been subjected to the following treatments as indicated: no treatment, 5 mM PMPS, addition of 10 mM DTT, and addition of 10 μM ZnCl2. The reaction products were then analyzed by native gel electrophoresis. (B) The Y substrate was incubated with 4 pmole of Slx1 or Slx4 that had been treated with 0, 0.2, 0.6, 1.0, 1.4, or 1.8 mM PMPS. Sub, substrate alone; Slx1–4, Slx1–4 complex with or without 0.2 mM PMPS treatment. (C) Strain NJY420 [sgs1-3:TRP1 slx1-11::HIS3 pJM500 (SGS1/URA3)] was transformed with either vector alone (pRS415) or vector containing SLX1 (pKR6129) or slx1-C241Y (pKR6128). Transformants were then streaked on media containing 5-FOA to select against pJM500. Plates were photographed following growth at 30°C for 3 d.
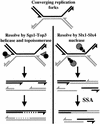
Figure 7.
Model of Sgs1–Top3 and Slx1–4 at the termination of rDNA replication. (Left) When replication forks converge and stall, Sgs1–Top3 helicase–topoisomerase separates the parental strands of the DNA template, allowing replication to finish by a DNA polymerase filling-in reaction (broken lines). (Right) In the absence of Sgs1–Top3, Slx1–4 cleaves the 5′ arm of each replication fork, creating a doubly nicked chromatid and a broken sister chromatid with 3′ overhangs. At the rDNA, this DSB can be repaired by_RAD52_-independent single-strand annealing (SSA). Arrowheads, 3′-ends.
Similar articles
- Functional overlap between Sgs1-Top3 and the Mms4-Mus81 endonuclease.
Kaliraman V, Mullen JR, Fricke WM, Bastin-Shanower SA, Brill SJ. Kaliraman V, et al. Genes Dev. 2001 Oct 15;15(20):2730-40. doi: 10.1101/gad.932201. Genes Dev. 2001. PMID: 11641278 Free PMC article. - Yeast Rmi1/Nce4 controls genome stability as a subunit of the Sgs1-Top3 complex.
Mullen JR, Nallaseth FS, Lan YQ, Slagle CE, Brill SJ. Mullen JR, et al. Mol Cell Biol. 2005 Jun;25(11):4476-87. doi: 10.1128/MCB.25.11.4476-4487.2005. Mol Cell Biol. 2005. PMID: 15899853 Free PMC article. - Requirement for three novel protein complexes in the absence of the Sgs1 DNA helicase in Saccharomyces cerevisiae.
Mullen JR, Kaliraman V, Ibrahim SS, Brill SJ. Mullen JR, et al. Genetics. 2001 Jan;157(1):103-18. doi: 10.1093/genetics/157.1.103. Genetics. 2001. PMID: 11139495 Free PMC article. - [Functional analysis of yeast homologue gene associated with human DNA helicase causative syndromes].
Miyajima A. Miyajima A. Kokuritsu Iyakuhin Shokuhin Eisei Kenkyusho Hokoku. 2002;(120):53-74. Kokuritsu Iyakuhin Shokuhin Eisei Kenkyusho Hokoku. 2002. PMID: 12638184 Review. Japanese. - Yeast as a model system to study RecQ helicase function.
Ashton TM, Hickson ID. Ashton TM, et al. DNA Repair (Amst). 2010 Mar 2;9(3):303-14. doi: 10.1016/j.dnarep.2009.12.007. Epub 2010 Jan 13. DNA Repair (Amst). 2010. PMID: 20071248 Review.
Cited by
- Assembly of Slx4 signaling complexes behind DNA replication forks.
Balint A, Kim T, Gallo D, Cussiol JR, Bastos de Oliveira FM, Yimit A, Ou J, Nakato R, Gurevich A, Shirahige K, Smolka MB, Zhang Z, Brown GW. Balint A, et al. EMBO J. 2015 Aug 13;34(16):2182-97. doi: 10.15252/embj.201591190. Epub 2015 Jun 25. EMBO J. 2015. PMID: 26113155 Free PMC article. - Structural and Mechanistic Analysis of the Slx1-Slx4 Endonuclease.
Gaur V, Wyatt HDM, Komorowska W, Szczepanowski RH, de Sanctis D, Gorecka KM, West SC, Nowotny M. Gaur V, et al. Cell Rep. 2015 Mar 10;10(9):1467-1476. doi: 10.1016/j.celrep.2015.02.019. Epub 2015 Mar 5. Cell Rep. 2015. PMID: 25753413 Free PMC article. - Mus81-Mms4 functions as a single heterodimer to cleave nicked intermediates in recombinational DNA repair.
Schwartz EK, Wright WD, Ehmsen KT, Evans JE, Stahlberg H, Heyer WD. Schwartz EK, et al. Mol Cell Biol. 2012 Aug;32(15):3065-80. doi: 10.1128/MCB.00547-12. Epub 2012 May 29. Mol Cell Biol. 2012. PMID: 22645308 Free PMC article. - HO Endonuclease-Initiated Recombination in Yeast Meiosis Fails To Promote Homologous Centromere Pairing and Is Not Constrained To Utilize the Dmc1 Recombinase.
Yisehak L, MacQueen AJ. Yisehak L, et al. G3 (Bethesda). 2018 Nov 6;8(11):3637-3659. doi: 10.1534/g3.118.200641. G3 (Bethesda). 2018. PMID: 30254180 Free PMC article. - Inferring transcriptional compensation interactions in yeast via stepwise structure equation modeling.
Shieh GS, Chen CM, Yu CY, Huang J, Wang WF, Lo YC. Shieh GS, et al. BMC Bioinformatics. 2008 Mar 3;9:134. doi: 10.1186/1471-2105-9-134. BMC Bioinformatics. 2008. PMID: 18312694 Free PMC article.
References
- Aasland R., Gibson, T.J., and Stewart, A.F. 1995. The PHD finger: Implications for chromatin-mediated transcriptional regulation. Trends Biochem. Sci. 20: 56–59. - PubMed
- Aravind L. and Koonin, E.V. 2000. SAP—A putative DNA-binding motif involved in chromosomal organization. Trends Biochem. Sci. 25: 112–114. - PubMed
- Bochkareva E., Frappier, L., Edwards, A.M., and Bochkarev, A. 1998. The RPA32 subunit of human replication protein A contains a single-stranded DNA-binding domain. J. Biol. Chem. 273: 3932–3936. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases