Rapid deadenylation triggered by a nonsense codon precedes decay of the RNA body in a mammalian cytoplasmic nonsense-mediated decay pathway - PubMed (original) (raw)
Rapid deadenylation triggered by a nonsense codon precedes decay of the RNA body in a mammalian cytoplasmic nonsense-mediated decay pathway
Chyi-Ying A Chen et al. Mol Cell Biol. 2003 Jul.
Abstract
Nonsense-mediated mRNA decay (NMD) is an RNA surveillance pathway that detects and destroys aberrant mRNAs containing nonsense or premature termination codons (PTCs) in a translation-dependent manner in eukaryotes. In yeast, the NMD pathway bypasses the deadenylation step and directly targets PTC-containing messages for decapping, followed by 5'-to-3' exonuclease digestion of the RNA body. In mammals, most PTC-containing mRNAs are subject to active nucleus-associated NMD. Here, using two distinct transcription-pulsing approaches to monitor mRNA deadenylation and decay kinetics, we demonstrate the existence of an active cytoplasmic NMD pathway in mammalian cells. In this pathway, a nonsense codon triggers accelerated deadenylation that precedes decay of the PTC-containing mRNA body. Transcript is stabilized when accelerated deadenylation is impeded by blocking translation initiation; by ectopically expressing two RNA-binding proteins, UNR and NSAP1; or by ectopically expressing a UPF1 dominant-negative mutant. These results are consistent with the notion that the nonsense codon can function in the cytoplasm by promoting rapid removal of the poly(A) tail as a necessary first step in the decay process.
Figures
FIG. 1.
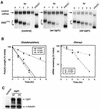
Nonsense codon triggers accelerated deadenylation of β-globin mRNA in the cytoplasm, preceding the decay of the mRNA body. (A) Physical map of β-globin gene whose transcription is driven by the c-fos or Tet-regulated promoter. The open rectangles, thin lines, and thick lines depict exons, introns, and the 3′ flanking region of the rabbit β-globin gene, respectively. The sequences encoding the translation initiation codon (ATG) and the termination codon (TGA) are indicated. The arrow marks the point where the PTC was introduced. (B) Left, Northern blots showing deadenylation and decay of wild-type (BBB) and PTC-containing (BBBPTC) β-globin mRNAs transcribed from the c-fos promoter in serum-induced NIH 3T3 cells. Middle, comparison of deadenylation rates of BBB and BBBPTC mRNAs. Right, comparison of decay rates of BBB and BBBPTC mRNAs. (C) Top, Northern blots showing deadenylation and decay of BBB and BBBPTC mRNAs transiently transcribed from the Tet-regulated promoter following a transcription pulse in proliferating NIH 3T3 B2_A_2 cells. Bottom left, comparison of deadenylation rates of BBB and BBBPTC mRNAs. Bottom right, comparison of decay rates of BBB and BBBPTC mRNAs. (B and C) NIH 3T3 cells (B) or subline cells stably harboring tTA for the Tet-regulated promoter system (C) were transiently cotransfected with a control plasmid (pSVα/GAPDH) and one of the test plasmids (pBBB or pBBBPTC). Total cytoplasmic mRNA was isolated at various time intervals after serum stimulation of quiescent cells (B) or after the addition of Tet (C) to proliferating cells and analyzed by Northern blot analysis (see Materials and Methods). α-globin/GAPDH control mRNAs were expressed constitutively and served as an internal standard. The times given above the blots correspond to hours after serum stimulation (B) or after Tet addition (C). Poly(A)− RNA was prepared in vitro by treating RNA samples with oligo(dT) and RNase H. Poly(A) lengths were compared by calculating from the difference in electrophoretic mobility between each message and cognate poly(A)− RNA. The quantitation of data was obtained by scanning the radioactive blots with an imager (Packard). Deadenylation and decay curves were plotted as described previously (34, 36). All curves shown in the plots are based on the average results of multiple experiments with standard deviations.
FIG. 2.
Mode of transcription of PTC-containing β-globin gene affects nonsense-mediated decay for the transcript. (A) Steady-state pool of cytoplasmic BBBPTC transcript decays at a rate identical to that of the normal BBB mRNA after being constitutively expressed. The Tet-regulated promoter-driven BBB and BBBPTC constructs were transiently transfected into NIH 3T3 B2_A_2 cells cultured in the absence of tetracycline to allow constitutive expression of BBB and BBBPTC transcripts following transfection. Thirty-six hours later, mRNA stability was determined by blocking ongoing transcription with tetracycline. RNA isolation, data quantitation, and plotting were as described in the legend to Fig. 1. Left, Northern blots showing decay of the cytoplasmic steady-state pool of BBB (wild-type) or BBBPTC (PTC) mRNA constitutively transcribed from the Tet-regulated promoter in proliferating NIH 3T3 B2_A_2 cells. Right, comparison of decay rates of BBB and BBBPTC mRNAs. (B) Northern blots showing the direct side-by-side comparison of the levels of wild-type (wt) BBB and BBBPTC transcripts transcribed from the Tet-regulated promoter in a transient (left) or constitutive (right) mode. Transfection, RNA isolation, and data quantitation were as described in Materials and Methods and in the legend to Fig. 1.
FIG. 3.
Targeted blockage of translation of PTC-containing β-globin mRNA impedes the deadenylation step and stabilizes the message. (A) Top, Northern blots showing deadenylation and decay of BBBPTC and BBBPTC+hp mRNAs. Bottom, comparison of deadenylation and decay rates of BBBPTC and BBBPTC+hp mRNAs. (B) Northern blots showing deadenylation and decay of BBB and BBB+hp mRNAs. Transient transfection, time course experiments, RNA isolation, poly(A)− RNA preparation, poly(A) length comparisons, and data quantitation and plotting were as described in the legend to Fig. 1. (Note: deadenylation and decay curves for BBBPTC mRNA are based on the results of multiple experiments, including the ones shown in this figure and in Fig. 1.)
FIG. 4.
Ectopic expression of a dominant-negative mutant of human UPF1 protein slows down deadenylation of PTC-containing β-globin mRNA, leading to message stabilization. (A) Northern blots showing deadenylation and decay of BBBPTC mRNA in proliferating NIH 3T3 cells in the absence (control) or presence of ectopically expressed wild-type (wt) or R844C mutant (mt) hUpf1 protein. (B) Comparison of deadenylation and decay rates of BBBPTC mRNA in the absence (control) or presence of ectopically expressed wt Upf1 or mt Upf1 protein. Transient transfection, time course experiments, RNA isolation, poly(A)− RNA preparation, poly(A) length comparisons, and data quantitation and plotting were as described in the legend to Fig. 1. (C) Western blot analysis showing that wt and mt Upf1 proteins were expressed at similar levels. Cytoplasmic lysates prepared from NIH 3T3 B2_A_2 cells transfected with BBBPTC in the absence (control) or presence of either wt Upf1 or mt Upf1 protein were resolved on a sodium dodecyl sulfate-8% polyacrylamide gel. The blot was probed with antibody against the Flag tag for detection of Upf1 and its mutant proteins and a control monoclonal antibody against α-tubulin for equal loading of protein samples.
FIG. 5.
Ectopic expression of UNR and NSAP1 blocks deadenylation of PTC-containing β-globin mRNA, leading to message stabilization. Shown are Northern blots demonstrating deadenylation and decay of BBBPTC mRNAs in serum-stimulated NIH 3T3 cells in the absence (B, Control) or presence of ectopically expressed UNR (A, top left), NSAP1 (A, top right), or HuR (B, bottom) and deadenylation and decay of BBB mRNA in the presence of ectopically expressed UNR (A, bottom left) or NSAP1 (A, bottom right). Transient transfection, time course experiments, RNA isolation, and poly(A)− RNA preparation were as described in the legend to Fig. 1.
Similar articles
- Proximity of the poly(A)-binding protein to a premature termination codon inhibits mammalian nonsense-mediated mRNA decay.
Silva AL, Ribeiro P, Inácio A, Liebhaber SA, Romão L. Silva AL, et al. RNA. 2008 Mar;14(3):563-76. doi: 10.1261/rna.815108. Epub 2008 Jan 29. RNA. 2008. PMID: 18230761 Free PMC article. - Processing bodies are not required for mammalian nonsense-mediated mRNA decay.
Stalder L, Mühlemann O. Stalder L, et al. RNA. 2009 Jul;15(7):1265-73. doi: 10.1261/rna.1672509. Epub 2009 May 27. RNA. 2009. PMID: 19474145 Free PMC article. - Nonsense-containing mRNAs that accumulate in the absence of a functional nonsense-mediated mRNA decay pathway are destabilized rapidly upon its restitution.
Maderazo AB, Belk JP, He F, Jacobson A. Maderazo AB, et al. Mol Cell Biol. 2003 Feb;23(3):842-51. doi: 10.1128/MCB.23.3.842-851.2003. Mol Cell Biol. 2003. PMID: 12529390 Free PMC article. - [Nonsense-mediated mRNA decay (NMD)--on guard of mRNA quality].
Dzikiewicz A, Szweykowska-Kulińska Z. Dzikiewicz A, et al. Postepy Biochem. 2006;52(4):390-8. Postepy Biochem. 2006. PMID: 17536508 Review. Polish. - Cutting the nonsense: the degradation of PTC-containing mRNAs.
Nicholson P, Mühlemann O. Nicholson P, et al. Biochem Soc Trans. 2010 Dec;38(6):1615-20. doi: 10.1042/BST0381615. Biochem Soc Trans. 2010. PMID: 21118136 Review.
Cited by
- SMG5-PNRC2 is functionally dominant compared with SMG5-SMG7 in mammalian nonsense-mediated mRNA decay.
Cho H, Han S, Choe J, Park SG, Choi SS, Kim YK. Cho H, et al. Nucleic Acids Res. 2013 Jan;41(2):1319-28. doi: 10.1093/nar/gks1222. Epub 2012 Dec 11. Nucleic Acids Res. 2013. PMID: 23234702 Free PMC article. - Nonsense-mediated RNA decay regulation by cellular stress: implications for tumorigenesis.
Gardner LB. Gardner LB. Mol Cancer Res. 2010 Mar;8(3):295-308. doi: 10.1158/1541-7786.MCR-09-0502. Epub 2010 Feb 23. Mol Cancer Res. 2010. PMID: 20179151 Free PMC article. Review. - Comparison of EJC-enhanced and EJC-independent NMD in human cells reveals two partially redundant degradation pathways.
Metze S, Herzog VA, Ruepp MD, Mühlemann O. Metze S, et al. RNA. 2013 Oct;19(10):1432-48. doi: 10.1261/rna.038893.113. Epub 2013 Aug 20. RNA. 2013. PMID: 23962664 Free PMC article. - SMG6 promotes endonucleolytic cleavage of nonsense mRNA in human cells.
Eberle AB, Lykke-Andersen S, Mühlemann O, Jensen TH. Eberle AB, et al. Nat Struct Mol Biol. 2009 Jan;16(1):49-55. doi: 10.1038/nsmb.1530. Epub 2008 Dec 7. Nat Struct Mol Biol. 2009. PMID: 19060897 - SMG6 is the catalytic endonuclease that cleaves mRNAs containing nonsense codons in metazoan.
Huntzinger E, Kashima I, Fauser M, Saulière J, Izaurralde E. Huntzinger E, et al. RNA. 2008 Dec;14(12):2609-17. doi: 10.1261/rna.1386208. Epub 2008 Oct 30. RNA. 2008. PMID: 18974281 Free PMC article.
References
- Beelman, C. A., and R. Parker. 1995. Degradation of mRNA in eukaryotes. Cell 81:179-183. - PubMed
- Bogdan, J. A., C. Adams-Burton, D. L. Pedicord, D. A. Sukovich, P. A. Benfield, M. H. Corjay, J. K. Stoltenborg, and I. B. Dicker. 1998. Human carbon catabolite repressor protein (CCR4)-associative factor 1: cloning, expression and characterization of its interaction with the B-cell translocation protein BTG1. Biochem. J. 336:471-481. - PMC - PubMed
- Cao, D., and R. Parker. 2003. Computational modeling and experimental analysis of nonsense-mediated decay in yeast. Cell 113:533-545. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources