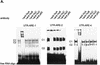Nitric oxide increases the decay of matrix metalloproteinase 9 mRNA by inhibiting the expression of mRNA-stabilizing factor HuR - PubMed (original) (raw)
Nitric oxide increases the decay of matrix metalloproteinase 9 mRNA by inhibiting the expression of mRNA-stabilizing factor HuR
El-Sayed Akool et al. Mol Cell Biol. 2003 Jul.
Abstract
Dysregulation of extracellular matrix turnover is an important feature of many inflammatory processes. Rat renal mesangial cells express high levels of matrix metalloproteinase 9 (MMP-9) in response to inflammatory cytokines such as interleukin-1 beta. We demonstrate that NO does strongly destabilize MMP-9 mRNA, since different luciferase reporter gene constructs containing the MMP-9 3' untranslated region (UTR) displayed significant reduced luciferase activity in response to the presence of NO. Moreover, by use of an in vitro degradation assay we found that the cytoplasmic fractions of NO-treated cells contained a higher capacity to degrade MMP-9 transcripts than those obtained from control cells. An RNA electrophoretic mobility shift assay demonstrated that three of four putative AU-rich elements present in the 3' UTR of MMP-9 were constitutively occupied by the mRNA-stabilizing factor HuR and that the RNA binding was strongly attenuated by the presence of NO. The addition of recombinant glutathione transferase-HuR prevented the rapid decay of MMP-9 mRNA, whereas the addition of a neutralizing anti-HuR antibody caused an acceleration of MMP-9 mRNA degradation. Furthermore, the expression of HuR mRNA and protein was significantly reduced by exogenously and endogenously produced NO. These inhibitory effects were mimicked by the cGMP analog 8-bromo-cGMP and reversed by LY-83583, an inhibitor of soluble guanylyl cyclase. These results demonstrate that NO acts in a cGMP-dependent mechanism to inhibit the expression level of HuR, thereby reducing the stability of MMP-9 mRNA.
Figures
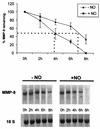
FIG. 1.
Accelerated degradation of cytokine-induced MMP-9 mRNA by DETA NONOate. Quiescent MC were treated for 20 h with IL-1β (2 nM) and then washed twice before the addition of actinomycin D (5 μg/ml). After a short preincubation of 30 min, cells were additionally treated for the indicated time points without (−NO) or with (+NO) DETA NONOate (500 μM) before being harvested and extracted for total cellular RNA. For Northern blot analysis, 20 μg of total cellular RNA was hybridized to a 32P-labeled MMP-9 probe. The equivalent loading of RNA was ascertained by subsequent hybridization with an 18S rRNA probe. The results of a representative experiment are shown in the lower part of the figure. The upper panel shows means ± standard deviations (n = 3) of three independent experiments and depicts the percentages of remaining MMP-9 transcripts after addition of DETA NONOate (NO).
FIG. 2.
Sequence of the 3′ UTR of the rat MMP-9 gene (GenBank accession no. U24441). Potential ARE binding sites involved in the posttranscriptional regulation of MMP-9 mRNA are depicted in boxes. The numbers of AREs were used to distinguish between the different ARE motifs within the MMP-9 UTR. The underlined sequences indicate the regions encompassed by the RNA oligonucleotides used for EMSA.
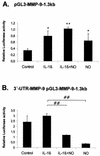
FIG. 3.
Influence of the 3′ UTR of MMP-9 on MMP-9 promoter-driven luciferase activities. Subconfluent MC were transiently cotransfected with 0.4 μg of pGL-MMP-9-1.3kb (A) or alternatively with 0.4 μg of 3′-UTR-MMP-9 pGL-MMP-9-1.3kb (B) containing in addition the 3′ UTR of MMP-9 downstream of the luciferase coding sequence. For each transfection, 0.1 μg of pRL-CMV coding for Renilla luciferase was simultaneously added. After an overnight transfection, MC were treated for 24 h with vehicle (control) or with IL-1β (2 nM) in the presence or absence of DETA NONOate (500 μM) as indicated. The values for beetle luciferase were related to values for Renilla luciferase and are depicted as relative luciferase activities. Data (means ± SEM) represent the results of three different experiments. P values were 0.05 and 0.01 versus control conditions (∗ and ∗∗, respectively) and 0.01 versus IL-1β-stimulated conditions (##).
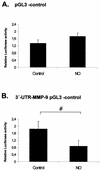
FIG. 4.
Influence of the 3′ UTR of MMP-9 on simian virus 40-driven luciferase activities. Subconfluent MC were transiently cotransfected with 0.4 μg of empty pGL3 control vector (A) or with 0.4 μg of 3′-UTR-MMP-9 pGL3 control (B) additionally containing the 3′ UTR of MMP-9 downstream of the luciferase coding sequence. Each plasmid was cotransfected with 0.1 μg of pRL-CMV used to equilibrate differences in transfection efficiencies. After transfection, MC were treated for 24 h with vehicle or with DETA NONOate (500 μM) as indicated. Values for beetle luciferase were related to values for Renilla luciferase and are depicted as relative luciferase activities. Data (means ± SEM) represent the results of three different experiments. P was 0.05 versus control conditions (#).
FIG. 5.
(A) DETA NONOate (DETA-NO) inhibits the constitutive RNA binding of cytoplasmic complexes to different ARE motifs from the 3′ UTR of MMP-9. RNA binding was analyzed by EMSAs using gene-specific oligonucleotides (Table 1). MC were either left untreated (−) or stimulated for 4 h with the indicated concentrations of DETA NONOate before being lysed for preparation of cytoplasmic extracts. Equal amounts of cytoplasmic extracts were incubated with a 32P-labeled RNA probe derived from the corresponding AU-rich region of the 3′ UTR of rat MMP-9 (UTR-ARE-1, UTR-ARE-2, and UTR-ARE-4), and RNA binding was assessed by using 6.0% native polyacrylamide electrophoresis gels. C1 and C2 indicate two main complexes constitutively binding to UTR-ARE-1, UTR-ARE-2, and UTR-ARE-4. The results shown in each panel are representative of three independent experiments giving similar results. (B) Cytokine-mediated inhibition of constitutive RNA binding to different MMP-9-specific AREs is attenuated in the presence of L-NMMA. MC were either left untreated (control) or stimulated for 24 h with IL-1β (2 nM), L-NMMA (2 mM), or both in combination as indicated before being collected for extraction. Equal amounts of cytoplasmic extracts were incubated with the 32P-labeled RNA probes specific for the 3′ UTR of rat MMP-9 depicted in Table 1. The conditions used for EMSA were as described for panel A. The results shown represent one experiment out of three giving similar results.
FIG. 6.
HuR (HuA) is the main constituent of RNA-binding complexes within the cytoplasmic extracts of MC and binds with a high affinity to AREs of the 3′ UTR of MMP-9. (A) For supershift analysis, the 32P-radiolabeled UTR-ARE-1-, UTR-ARE-2-, and UTR-ARE-4-comprising oligonucleotides were incubated with 6 μg of cytoplasmic extract derived from resting MC in the presence or absence of different supershift antibodies as indicated. The antibodies were added 15 min after the addition of the radiolabeled oligonucleotide and incubatedfor a further 15 min at room temperature. (B) Competition capacities of wild-type and mutant UTR-ARE-1 oligonucleotides. The gel shows the results of a representative competition study using different dilutions (as indicated) of either unlabeled wild-type (UTR-ARE-1wt) or unlabeled mutated ARE-1 (UTR-ARE-1mut) oligonucleotides (the sequences are shown in Table 1). The unlabeled competitor oligonucleotides were added 60 min before the addition of 32P-labeled UTR-ARE-1 wild-type oligonucleotide. (C and D) GST-HuR (C) but not GST (D) binds with a high affinity to AREs of the 3′ UTR of MMP-9. The results of EMSA analysis are shown, demonstrating that recombinant GST-HuR protein binds with high affinity to AREs of the 3′-UTR of MMP-9 (C) whereas GST alone has only a weak unspecific RNA binding affinity (D). For EMSA, 200 ng of purified crude protein (GST-HuR or, alternatively, GST) was incubated with the indicated oligonucleotides specific for different AREs of MMP-9 as described in Materials and Methods. For supershift analysis, the 32P-radiolabeled UTR-ARE-1-, UTR-ARE-2-, and UTR-ARE-4-comprising oligonucleotides were incubated with the identical amounts of GST-HuR protein and 200 ng of supershift antibody as indicated. The antibodies were added 15 min after addition of the radiolabeled oligonucleotide and incubated for a further 15 min at room temperature. Similar results were obtained in three independent experiments.
FIG. 6.
HuR (HuA) is the main constituent of RNA-binding complexes within the cytoplasmic extracts of MC and binds with a high affinity to AREs of the 3′ UTR of MMP-9. (A) For supershift analysis, the 32P-radiolabeled UTR-ARE-1-, UTR-ARE-2-, and UTR-ARE-4-comprising oligonucleotides were incubated with 6 μg of cytoplasmic extract derived from resting MC in the presence or absence of different supershift antibodies as indicated. The antibodies were added 15 min after the addition of the radiolabeled oligonucleotide and incubatedfor a further 15 min at room temperature. (B) Competition capacities of wild-type and mutant UTR-ARE-1 oligonucleotides. The gel shows the results of a representative competition study using different dilutions (as indicated) of either unlabeled wild-type (UTR-ARE-1wt) or unlabeled mutated ARE-1 (UTR-ARE-1mut) oligonucleotides (the sequences are shown in Table 1). The unlabeled competitor oligonucleotides were added 60 min before the addition of 32P-labeled UTR-ARE-1 wild-type oligonucleotide. (C and D) GST-HuR (C) but not GST (D) binds with a high affinity to AREs of the 3′ UTR of MMP-9. The results of EMSA analysis are shown, demonstrating that recombinant GST-HuR protein binds with high affinity to AREs of the 3′-UTR of MMP-9 (C) whereas GST alone has only a weak unspecific RNA binding affinity (D). For EMSA, 200 ng of purified crude protein (GST-HuR or, alternatively, GST) was incubated with the indicated oligonucleotides specific for different AREs of MMP-9 as described in Materials and Methods. For supershift analysis, the 32P-radiolabeled UTR-ARE-1-, UTR-ARE-2-, and UTR-ARE-4-comprising oligonucleotides were incubated with the identical amounts of GST-HuR protein and 200 ng of supershift antibody as indicated. The antibodies were added 15 min after addition of the radiolabeled oligonucleotide and incubated for a further 15 min at room temperature. Similar results were obtained in three independent experiments.
FIG. 6.
HuR (HuA) is the main constituent of RNA-binding complexes within the cytoplasmic extracts of MC and binds with a high affinity to AREs of the 3′ UTR of MMP-9. (A) For supershift analysis, the 32P-radiolabeled UTR-ARE-1-, UTR-ARE-2-, and UTR-ARE-4-comprising oligonucleotides were incubated with 6 μg of cytoplasmic extract derived from resting MC in the presence or absence of different supershift antibodies as indicated. The antibodies were added 15 min after the addition of the radiolabeled oligonucleotide and incubatedfor a further 15 min at room temperature. (B) Competition capacities of wild-type and mutant UTR-ARE-1 oligonucleotides. The gel shows the results of a representative competition study using different dilutions (as indicated) of either unlabeled wild-type (UTR-ARE-1wt) or unlabeled mutated ARE-1 (UTR-ARE-1mut) oligonucleotides (the sequences are shown in Table 1). The unlabeled competitor oligonucleotides were added 60 min before the addition of 32P-labeled UTR-ARE-1 wild-type oligonucleotide. (C and D) GST-HuR (C) but not GST (D) binds with a high affinity to AREs of the 3′ UTR of MMP-9. The results of EMSA analysis are shown, demonstrating that recombinant GST-HuR protein binds with high affinity to AREs of the 3′-UTR of MMP-9 (C) whereas GST alone has only a weak unspecific RNA binding affinity (D). For EMSA, 200 ng of purified crude protein (GST-HuR or, alternatively, GST) was incubated with the indicated oligonucleotides specific for different AREs of MMP-9 as described in Materials and Methods. For supershift analysis, the 32P-radiolabeled UTR-ARE-1-, UTR-ARE-2-, and UTR-ARE-4-comprising oligonucleotides were incubated with the identical amounts of GST-HuR protein and 200 ng of supershift antibody as indicated. The antibodies were added 15 min after addition of the radiolabeled oligonucleotide and incubated for a further 15 min at room temperature. Similar results were obtained in three independent experiments.
FIG. 6.
HuR (HuA) is the main constituent of RNA-binding complexes within the cytoplasmic extracts of MC and binds with a high affinity to AREs of the 3′ UTR of MMP-9. (A) For supershift analysis, the 32P-radiolabeled UTR-ARE-1-, UTR-ARE-2-, and UTR-ARE-4-comprising oligonucleotides were incubated with 6 μg of cytoplasmic extract derived from resting MC in the presence or absence of different supershift antibodies as indicated. The antibodies were added 15 min after the addition of the radiolabeled oligonucleotide and incubatedfor a further 15 min at room temperature. (B) Competition capacities of wild-type and mutant UTR-ARE-1 oligonucleotides. The gel shows the results of a representative competition study using different dilutions (as indicated) of either unlabeled wild-type (UTR-ARE-1wt) or unlabeled mutated ARE-1 (UTR-ARE-1mut) oligonucleotides (the sequences are shown in Table 1). The unlabeled competitor oligonucleotides were added 60 min before the addition of 32P-labeled UTR-ARE-1 wild-type oligonucleotide. (C and D) GST-HuR (C) but not GST (D) binds with a high affinity to AREs of the 3′ UTR of MMP-9. The results of EMSA analysis are shown, demonstrating that recombinant GST-HuR protein binds with high affinity to AREs of the 3′-UTR of MMP-9 (C) whereas GST alone has only a weak unspecific RNA binding affinity (D). For EMSA, 200 ng of purified crude protein (GST-HuR or, alternatively, GST) was incubated with the indicated oligonucleotides specific for different AREs of MMP-9 as described in Materials and Methods. For supershift analysis, the 32P-radiolabeled UTR-ARE-1-, UTR-ARE-2-, and UTR-ARE-4-comprising oligonucleotides were incubated with the identical amounts of GST-HuR protein and 200 ng of supershift antibody as indicated. The antibodies were added 15 min after addition of the radiolabeled oligonucleotide and incubated for a further 15 min at room temperature. Similar results were obtained in three independent experiments.
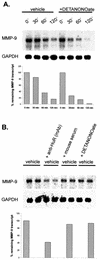
FIG.7.
HuR protects MMP-9 mRNA from rapid decay within the cytoplasmic fractions of MC. (A) For in vitro degradation assays, portions of 20 μg of total RNA from a common pool of total RNA isolated from cytokine-stimulated MC were mixed with 130 μg of cytoplasmic extract derived from either untreated (vehicle) MC or, alternatively, MC treated for 4 h with DETA NONOate (+DETANONOate) (500 μM) as indicated. At different time points as indicated, incubations were stopped by isolation of total RNA. RNA samples were collected and assessed for the remaining amounts of MMP-9 mRNA by Northern blot analysis using a 32P-labeled cDNA insert specific for rat MMP-9. Furthermore, the blots were rehybridized with a 32P-labeled cDNA from GAPDH to prove the presence of equivalent mRNA amounts. The lower panel shows a densitometric analysis of MMP-9-specific signals. Similar results were obtained in three independent experiments. (B) The degradation of cytokine-induced MMP-9 transcripts is accelerated by a neutralizing anti-HuR antibody. Portions of 20 μg of total RNA from a common pool of total RNA isolated from cytokine-stimulated MC were mixed with 130 μg of cytoplasmic extract derived from untreated cells. The cytoplasmic extracts were either kept untreated (vehicle) or, alternatively, were pretreated for 1 h with 0.4 μg of a monoclonal anti-HuR antibody [vehicle + anti-HuR (mAb)], with the same volume of vehicle (vehicle + mouse serum), or with DETA NONOate (500 μM) (vehicle + DETANONOate) before the cytoplasmic extracts were incubated with the total RNA portions. Incubation with the RNA was stopped after 60 min before RNA was extracted for further Northern blot analysis. Samples derived from one cytoplasmic extract were subjected to RNA in duplicates. Furthermore, the blots were rehybridized with a 32P-labeled cDNA from GAPDH to prove the presence of equivalent mRNA amounts. The lower panel shows a densitometric analysis of MMP-9-specific signals. The results shown are representative of one experiment out of three giving similar results. (C) GST-HuR protein protects MMP-9 mRNA from rapid decay. Total RNA (20 μg) from cytokine-stimulated MC was mixed with 130 μg of cytoplasmic extract derived from untreated MC in the absence (vehicle) or presence of 200 ng of GST-HuR or GST. At the indicated time points (0 h and 2 h), incubations were stopped by isolation of total RNA. Subsequently, the RNA was assessed by Northern blot analysis using an MMP-9-specific probe. Furthermore, to prove the specificity of the decay of MMP-9 mRNA, blots were rehybridized with a 32P-labeled cDNA from GAPDH. (D) Neutralization of HuR but not that of other Hu family members leads to a reduction in MMP-9 mRNA stability. Portions of 20 μg of total RNA isolated from cytokine-stimulated MC were mixed with 130 μg of cytoplasmic extract derived from untreated cells. The cytoplasmic extracts were kept either untreated (vehicle) or pretreated for 1 h with 0.4 μg of the Hu-specific antibodies as indicated before the cytoplasmic extracts were incubated with the total RNA portions. Incubation with the RNA was stopped after 60 min before RNA was extracted for Northern blot analysis. Samples derived from one cytoplasmic extract were subjected to RNA in duplicates. To prove the specificity of the effects on MMP-9 mRNA, blots were rehybridized with a 32P-labeled cDNA from GAPDH. Similar results were obtained in two independent experiments.
FIG.7.
HuR protects MMP-9 mRNA from rapid decay within the cytoplasmic fractions of MC. (A) For in vitro degradation assays, portions of 20 μg of total RNA from a common pool of total RNA isolated from cytokine-stimulated MC were mixed with 130 μg of cytoplasmic extract derived from either untreated (vehicle) MC or, alternatively, MC treated for 4 h with DETA NONOate (+DETANONOate) (500 μM) as indicated. At different time points as indicated, incubations were stopped by isolation of total RNA. RNA samples were collected and assessed for the remaining amounts of MMP-9 mRNA by Northern blot analysis using a 32P-labeled cDNA insert specific for rat MMP-9. Furthermore, the blots were rehybridized with a 32P-labeled cDNA from GAPDH to prove the presence of equivalent mRNA amounts. The lower panel shows a densitometric analysis of MMP-9-specific signals. Similar results were obtained in three independent experiments. (B) The degradation of cytokine-induced MMP-9 transcripts is accelerated by a neutralizing anti-HuR antibody. Portions of 20 μg of total RNA from a common pool of total RNA isolated from cytokine-stimulated MC were mixed with 130 μg of cytoplasmic extract derived from untreated cells. The cytoplasmic extracts were either kept untreated (vehicle) or, alternatively, were pretreated for 1 h with 0.4 μg of a monoclonal anti-HuR antibody [vehicle + anti-HuR (mAb)], with the same volume of vehicle (vehicle + mouse serum), or with DETA NONOate (500 μM) (vehicle + DETANONOate) before the cytoplasmic extracts were incubated with the total RNA portions. Incubation with the RNA was stopped after 60 min before RNA was extracted for further Northern blot analysis. Samples derived from one cytoplasmic extract were subjected to RNA in duplicates. Furthermore, the blots were rehybridized with a 32P-labeled cDNA from GAPDH to prove the presence of equivalent mRNA amounts. The lower panel shows a densitometric analysis of MMP-9-specific signals. The results shown are representative of one experiment out of three giving similar results. (C) GST-HuR protein protects MMP-9 mRNA from rapid decay. Total RNA (20 μg) from cytokine-stimulated MC was mixed with 130 μg of cytoplasmic extract derived from untreated MC in the absence (vehicle) or presence of 200 ng of GST-HuR or GST. At the indicated time points (0 h and 2 h), incubations were stopped by isolation of total RNA. Subsequently, the RNA was assessed by Northern blot analysis using an MMP-9-specific probe. Furthermore, to prove the specificity of the decay of MMP-9 mRNA, blots were rehybridized with a 32P-labeled cDNA from GAPDH. (D) Neutralization of HuR but not that of other Hu family members leads to a reduction in MMP-9 mRNA stability. Portions of 20 μg of total RNA isolated from cytokine-stimulated MC were mixed with 130 μg of cytoplasmic extract derived from untreated cells. The cytoplasmic extracts were kept either untreated (vehicle) or pretreated for 1 h with 0.4 μg of the Hu-specific antibodies as indicated before the cytoplasmic extracts were incubated with the total RNA portions. Incubation with the RNA was stopped after 60 min before RNA was extracted for Northern blot analysis. Samples derived from one cytoplasmic extract were subjected to RNA in duplicates. To prove the specificity of the effects on MMP-9 mRNA, blots were rehybridized with a 32P-labeled cDNA from GAPDH. Similar results were obtained in two independent experiments.
FIG. 8.
Inhibition of HuR expression in MC by different NO donors. MC were treated for 4 h with different concentrations of either DETA NONOate (left panel) or SNAP (right panel) before cells were lysed for Western blot analysis (A) or for extraction of total RNA (B). (A) Total protein (50 μg) was subjected to Western blot analysis using a monoclonal antibody raised against full-length HuR. Migration properties were determined using standard molecular weight markers. The Western blots shown in panel A were stripped and rehybridized to assess total β-actin levels as a control for sample loading and protein transfer. Similar results were obtained in two independent experiments. (B) Northern blot analysis demonstrating a dose-dependent modulation of HuR mRNA steady-state levels by different NO donors. Total cellular RNA (20 μg) was hybridized to a 32P-labeled cDNA insert from the plasmid HuR9 and analyzed by Northern blot analysis. Equivalent loading of RNA was ascertained by rehybridization to an 18S ribosomal probe. The blot shown is representative of two independent experiments giving similar results.
FIG. 9.
Cytokine-mediated inhibition of steady-state level of HuR mRNA (A) and protein (B) is blocked in the presence of the NOS inhibitor L-NMMA. Quiescent MC were treated for 24 h with vehicle (control) or with IL-1β (2 nM) or L-NMMA (2 mM) or both in combination as indicated before being harvested for either total RNA isolation (A) or Western blot analysis (B). (A) Total RNA (20 μg) was hybridized to a 32P-labeled cDNA insert from the plasmid HuR9 and analyzed by Northern blot analysis. Equivalent loading of RNA was ascertained by rehybridization to an 18S ribosomal probe. The blot shown is representative of two independent experiments giving similar results. (B) Total protein (50 μg) was subjected to Western blot analysis using a monoclonal HuR-specific antibody. The Western blot shown in panel B was stripped and rehybridized to assess the total β-actin levels as a control for sample loading and protein transfer.
FIG. 10.
(A) Suppression of cellular HuR level by 8-bromo-cGMP. MC were treated for 4 h with vehicle (control; −) or with the different concentrations of the cGMP analog 8-bromo-cGMP as indicated before cells were lysed. Total protein (50 μg) was subjected to Western blot analysis using a monoclonal antibody raised against full-length HuR. Migration properties were determined using standard molecular weight markers. (B) The reduction of HuR protein levels by DETA NONOate is antagonized in the presence of the guanylyl cyclase inhibitor LY-83583. Quiescent MC were treated for 4 h with vehicle (control; −) or with DETA NONOate (500 μM) or LY-83583 (500 nM) or both in combination as indicated before being harvested for preparation of total protein extracts. The level of total cellular HuR content was assessed with a monoclonal anti-HuR antibody. All Western blots were finally stripped and rehybridized to assess total β-actin levels as a control for sample loading and protein transfer. Similar results were obtained in two independent experiments.
Similar articles
- ATP potentiates interleukin-1 beta-induced MMP-9 expression in mesangial cells via recruitment of the ELAV protein HuR.
Huwiler A, Akool el-S, Aschrafi A, Hamada FM, Pfeilschifter J, Eberhardt W. Huwiler A, et al. J Biol Chem. 2003 Dec 19;278(51):51758-69. doi: 10.1074/jbc.M305722200. Epub 2003 Oct 1. J Biol Chem. 2003. PMID: 14523003 - Down-regulation of soluble guanylyl cyclase expression by cyclic AMP is mediated by mRNA-stabilizing protein HuR.
Klöss S, Srivastava R, Mülsch A. Klöss S, et al. Mol Pharmacol. 2004 Jun;65(6):1440-51. doi: 10.1124/mol.65.6.1440. Mol Pharmacol. 2004. PMID: 15155837 - H2O2 regulation of vascular function through sGC mRNA stabilization by HuR.
Martín-Garrido A, González-Ramos M, Griera M, Guijarro B, Cannata-Andia J, Rodriguez-Puyol D, Rodriguez-Puyol M, Saura M. Martín-Garrido A, et al. Arterioscler Thromb Vasc Biol. 2011 Mar;31(3):567-73. doi: 10.1161/ATVBAHA.110.219725. Epub 2010 Dec 16. Arterioscler Thromb Vasc Biol. 2011. PMID: 21164076 - Post-transcriptional regulation of soluble guanylyl cyclase expression in rat aorta.
Kloss S, Furneaux H, Mülsch A. Kloss S, et al. J Biol Chem. 2003 Jan 24;278(4):2377-83. doi: 10.1074/jbc.M206453200. Epub 2002 Nov 18. J Biol Chem. 2003. PMID: 12441354 - HuR and mRNA stability.
Brennan CM, Steitz JA. Brennan CM, et al. Cell Mol Life Sci. 2001 Feb;58(2):266-77. doi: 10.1007/PL00000854. Cell Mol Life Sci. 2001. PMID: 11289308 Free PMC article. Review.
Cited by
- Collapsed Reticular Network and its Possible Mechanism during the Initiation and/or Progression of Hepatic Fibrosis.
Wen SL, Feng S, Tang SH, Gao JH, Zhang LH, Tong H, Yan ZP, Fang DZ. Wen SL, et al. Sci Rep. 2016 Oct 14;6:35426. doi: 10.1038/srep35426. Sci Rep. 2016. PMID: 27739503 Free PMC article. - Expression Profile of Six RNA-Binding Proteins in Pulmonary Sarcoidosis.
Navratilova Z, Novosadova E, Hagemann-Jensen M, Kullberg S, Kolek V, Grunewald J, Petrek M. Navratilova Z, et al. PLoS One. 2016 Aug 30;11(8):e0161669. doi: 10.1371/journal.pone.0161669. eCollection 2016. PLoS One. 2016. PMID: 27575817 Free PMC article. - Endothelin-1 expression is strongly repressed by AU-rich elements in the 3'-untranslated region of the gene.
Reimunde FM, Castañares C, Redondo-Horcajo M, Lamas S, Rodríguez-Pascual F. Reimunde FM, et al. Biochem J. 2005 May 1;387(Pt 3):763-72. doi: 10.1042/BJ20041687. Biochem J. 2005. PMID: 15595926 Free PMC article. - Research progress on RNA-binding proteins in breast cancer.
Zhang W, Liu L, Zhao S, Chen L, Wei Y, Chen W, Ge F. Zhang W, et al. Oncol Lett. 2022 Apr;23(4):121. doi: 10.3892/ol.2022.13241. Epub 2022 Feb 14. Oncol Lett. 2022. PMID: 35261635 Free PMC article. Review. - The Triple Crown: NO, CO, and H2S in cancer cell biology.
Oza PP, Kashfi K. Oza PP, et al. Pharmacol Ther. 2023 Sep;249:108502. doi: 10.1016/j.pharmthera.2023.108502. Epub 2023 Jul 28. Pharmacol Ther. 2023. PMID: 37517510 Free PMC article. Review.
References
- Abdelaziz, N., F. Colombo, I. Mercier, and A. Calderone. 2001. Nitric oxide attenuates the expression of transforming growth factor-β (3) mRNA in rat cardiac fibroblasts via destabilization. Hypertension 38:261-266. - PubMed
- Atasoy, U., J. Watson, D. Patel, and J. D. Keene. 1998. ELAV protein HuA (HuR) can redistribute between nucleus and cytoplasm and is upregulated during serum stimulation and T cell activation. J. Cell Sci. 111:3145-3156. - PubMed
- Biragyn, A., and S. A. Nedospasov. 1995. Lipopolysaccharide-induced expression of TNFα gene in the macrophage cell line ANA-1 is regulated at the level of transcription processivity. J. Immunol. 155:674-683. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous