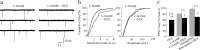Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism - PubMed (original) (raw)
Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism
Shi Di et al. J Neurosci. 2003.
Abstract
Glucocorticoid negative feedback in the brain controls stress, feeding, and neural-immune interactions by regulating the hypothalamic-pituitary-adrenal axis, but the mechanisms of inhibition of hypothalamic neurosecretory cells have never been elucidated. Using whole-cell patch-clamp recordings in an acute hypothalamic slice preparation, we demonstrate a rapid suppression of excitatory glutamatergic synaptic inputs to parvocellular neurosecretory neurons of the hypothalamic paraventricular nucleus (PVN) by the glucocorticoids dexamethasone and corticosterone. The effect was maintained with dexamethasone conjugated to bovine serum albumin and was not seen with direct intracellular glucocorticoid perfusion via the patch pipette, suggesting actions at a membrane receptor. The presynaptic inhibition of glutamate release by glucocorticoids was blocked by postsynaptic inhibition of G-protein activity with intracellular GDP-beta-S application, implicating a postsynaptic G-protein-coupled receptor and the release of a retrograde messenger. The glucocorticoid effect was not blocked by the nitric oxide synthesis antagonist N(G)-nitro-L-arginine methyl ester hydrochloride or by hemoglobin but was blocked completely by the CB1 cannabinoid receptor antagonists AM251 [N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide] and AM281 [1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-4-morpholinyl-1H-pyrazole-3-carboxamide] and mimicked and occluded by the cannabinoid receptor agonist WIN55,212-2 [(beta)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate], indicating that it was mediated by retrograde endocannabinoid release. Several peptidergic subtypes of parvocellular neuron, identified by single-cell reverse transcripton-PCR analysis, were subject to rapid inhibitory glucocorticoid regulation, including corticotropin-releasing hormone-, thyrotropin-releasing hormone-, vasopressin-, and oxytocin-expressing neurons. Therefore, our findings reveal a mechanism of rapid glucocorticoid feedback inhibition of hypothalamic hormone secretion via endocannabinoid release in the PVN and provide a link between the actions of glucocorticoids and cannabinoids in the hypothalamus that regulate stress and energy homeostasis.
Figures
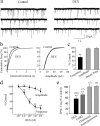
Figure 1.
Glucocorticoids inhibited glutamate release onto PVN parvocellular neurons. a, Bath application of DEX (1 μ
m
) elicited a decrease in the frequency of mEPSCs. b, Cumulative frequency plots of mEPSC interval and amplitude distributions from the same cell showed a significant reduction in mEPSC frequency (p < 0.01) with no change in mEPSC amplitude (p = 0.28). c, Mean changes in the average mEPSC frequency, amplitude, and decay time in dexamethasone (1 μ
m
) in 18 PVN parvocellular neurons. d, The dexamethasone-induced decrease in mEPSC frequency was dose dependent. e, Corticosterone (CORT, 1 μ
m
) caused a similar decrease in the mean frequency of mEPSCs, whereas the corticosteroid precursor cholesterol (5 μ
m
) and the physiologically inactive steroid isopregnanolone (5 μ
m
) had no effect on mEPSC frequency. Numbers in parentheses represent numbers of cells analyzed in each condition in this and in the following figures. *p < 0.05; **p < 0.01.
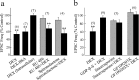
Figure 2.
The glucocorticoid effect was mediated by a postsynaptic, G-protein-coupled membrane receptor. a, Bath application of membrane-impermeant DEX—BSA maintained the inhibitory effect, whereas intracellular application of dexamethasone (1 μ
m
) had no effect on the frequency of mEPSCs. The intracellular glucocorticoid receptor antagonist RU486 (10 μ
m
) and the intracellular mineralocorticoid receptor antagonist spironolactone (10 μ
m
) failed to block the inhibitory effect of dexamethasone on mEPSCs.b, G-protein and protein-kinase blockers abolished the effect of glucocorticoid on mEPSCs. Postsynaptic G-protein blockade with intracellular GDP-β-S application (500 μ
m
) blocked the effect of dexamethasone on mEPSCs, implicating a postsynaptic G-protein-coupled mechanism. Kinase inhibition with bath application of staurosporine (0.5 μ
m
) or a PKC blocker, GF109203X (0.5 μ
m
), also abolished the inhibitory effect of dexamethasone on mEPSCs, suggesting a PKC-dependent mechanism that is either presynaptic or postsynaptic. **p < 0.01.
Figure 3.
Glucocorticoid inhibition of glutamate release was not mediated by NO or CO. a, The dexamethasone-induced suppression of mEPSCs was not blocked by the previous bath application of the NOS inhibitor
l
-NAME (10 μ
m
). b, Cumulative frequency plots showed a significant reduction in mEPSC frequency (p < 0.05) with no change in mEPSC amplitude (p = 0.24) in the presence of
l
-NAME and DEX. c, The NO—CO scavenger hemoglobin (10 μ
m
) also failed to block the inhibitory effect of dexamethasone on the frequency of mEPSCs. *p < 0.05; **p < 0.01.
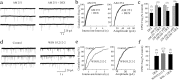
Figure 4.
The effect of glucocorticoid on glutamate release was mediated by an endocannabinoid. a, The dexamethasone-induced suppression of mEPSCs was prevented by previous bath application of the CB1 receptor antagonist AM251 (1 μ
m
). b, There was no effect of dexamethasone on the cumulative mEPSC interevent interval or amplitude distribution in the presence of AM251. c, Mean changes in average mEPSC frequency in the presence of cannabinoid receptor antagonists. The CB1 receptor antagonist AM251 blocked the effect of dexamethasone on mEPSC frequency. The CB2 receptor antagonist AM630 failed to block the DEX effect at a 1 μ
m
concentration but blocked the DEX effect at the higher, nonselective 10 μ
m
concentration. d, A cannabinoid receptor agonist, WIN55,212-2 (1 μ
m
), mimicked the glucocorticoid effect, causing a selective reduction in the frequency of mEPSCs. e, Cumulative frequency plots showed a significant increase in mEPSC interval (p < 0.01) but no change in mEPSC amplitude (p = 0.42) in WIN55,212-2. f, Mean changes in the average mEPSC frequency in the presence of glucocorticoid and the cannabinoid receptor agonist. The effect of WIN55,212-2 was similar to that of dexamethasone, but these effects were not additive, suggesting converging mechanisms. *p < 0.05; **p < 0.01.
Figure 5.
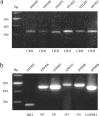
RT-PCR analysis in individual parvocellular neurons. a, CRH mRNA expression in six individual PVN parvocellular neurons. The expected CRH PCR product is 326 bp. b, TRH, VP, OT, and GAPDH mRNA expression in individual PVN parvocellular neurons. The expected PCR product weights are 276 bp (TRH), 440 bp (VP), 463 bp (OT) and 441 bp (GAPDH). The cell identification numbers are shown above each lane. A DNA ladder ranging from 300 to 500 bp is shown in the left lane of each panel.
Figure 6.
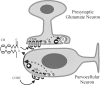
A model of the rapid glucocorticoid actions in PVN parvocellular neurons. The proposed mechanisms include glucocorticoid (CORT) binding to a G-protein-coupled glucocorticoid receptor (GRmb) and activation of an intracellular signaling pathway (dashed arrow) that leads to endocannabinoid synthesis (+). Endocannabinoid (CB) is released from the PVN neuron and binds to a G-protein-coupled CB1 cannabinoid receptor (CBR) on presynaptic glutamate terminals, activating a signaling cascade (dashed arrow) that leads to the inhibition of glutamate (GLU) release onto the PVN neuron-leading to decreased PVN neuronal activity and hormone secretion.
Similar articles
- Glucocorticoids regulate glutamate and GABA synapse-specific retrograde transmission via divergent nongenomic signaling pathways.
Di S, Maxson MM, Franco A, Tasker JG. Di S, et al. J Neurosci. 2009 Jan 14;29(2):393-401. doi: 10.1523/JNEUROSCI.4546-08.2009. J Neurosci. 2009. PMID: 19144839 Free PMC article. - Rapid glucocorticoid-mediated endocannabinoid release and opposing regulation of glutamate and gamma-aminobutyric acid inputs to hypothalamic magnocellular neurons.
Di S, Malcher-Lopes R, Marcheselli VL, Bazan NG, Tasker JG. Di S, et al. Endocrinology. 2005 Oct;146(10):4292-301. doi: 10.1210/en.2005-0610. Epub 2005 Jun 30. Endocrinology. 2005. PMID: 15994343 - Opposing crosstalk between leptin and glucocorticoids rapidly modulates synaptic excitation via endocannabinoid release.
Malcher-Lopes R, Di S, Marcheselli VS, Weng FJ, Stuart CT, Bazan NG, Tasker JG. Malcher-Lopes R, et al. J Neurosci. 2006 Jun 14;26(24):6643-50. doi: 10.1523/JNEUROSCI.5126-05.2006. J Neurosci. 2006. PMID: 16775153 Free PMC article. - Rapid synapse-specific regulation of hypothalamic magnocellular neurons by glucocorticoids.
Di S, Tasker JG. Di S, et al. Prog Brain Res. 2008;170:379-88. doi: 10.1016/S0079-6123(08)00431-7. Prog Brain Res. 2008. PMID: 18655897 Review. - Rapid glucocorticoid actions in the hypothalamus as a mechanism of homeostatic integration.
Tasker JG. Tasker JG. Obesity (Silver Spring). 2006 Aug;14 Suppl 5:259S-265S. doi: 10.1038/oby.2006.320. Obesity (Silver Spring). 2006. PMID: 17021378 Review.
Cited by
- Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance.
Liston C, Cichon JM, Jeanneteau F, Jia Z, Chao MV, Gan WB. Liston C, et al. Nat Neurosci. 2013 Jun;16(6):698-705. doi: 10.1038/nn.3387. Epub 2013 Apr 28. Nat Neurosci. 2013. PMID: 23624512 Free PMC article. - The thrifty lipids: endocannabinoids and the neural control of energy conservation.
DiPatrizio NV, Piomelli D. DiPatrizio NV, et al. Trends Neurosci. 2012 Jul;35(7):403-11. doi: 10.1016/j.tins.2012.04.006. Epub 2012 May 22. Trends Neurosci. 2012. PMID: 22622030 Free PMC article. Review. - Marijuana, endocannabinoids, and epilepsy: potential and challenges for improved therapeutic intervention.
Hofmann ME, Frazier CJ. Hofmann ME, et al. Exp Neurol. 2013 Jun;244:43-50. doi: 10.1016/j.expneurol.2011.11.047. Epub 2011 Dec 9. Exp Neurol. 2013. PMID: 22178327 Free PMC article. Review. - Neural regulation of the stress response: glucocorticoid feedback mechanisms.
Herman JP, McKlveen JM, Solomon MB, Carvalho-Netto E, Myers B. Herman JP, et al. Braz J Med Biol Res. 2012 Apr;45(4):292-8. doi: 10.1590/s0100-879x2012007500041. Epub 2012 Mar 29. Braz J Med Biol Res. 2012. PMID: 22450375 Free PMC article. Review. - Potential role of the endocannabinoid receptor antagonist rimonabant in the management of cardiometabolic risk: a narrative review of available data.
Bronander KA, Bloch MJ. Bronander KA, et al. Vasc Health Risk Manag. 2007;3(2):181-90. doi: 10.2147/vhrm.2007.3.2.181. Vasc Health Risk Manag. 2007. PMID: 17580728 Free PMC article. Review.
References
- Auclair N, Otani S, Soubrie P, Crepel F ( 2000) Cannabinoids modulate synaptic strength and plasticity at glutamatergic synapses of rat prefrontal cortex pyramidal neurons. J Neurophysiol 83: 3287–3293. - PubMed
- Avanzino GL, Ermirio R, Ruggeri P, Cogo CE ( 1987) Effects of corticosterone on neurons of reticular formation in rats. Am J Physiol 253: R25–R30. - PubMed
- Bredt DS, Hwang PM, Snyder SH ( 1990) Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature 347: 768–770. - PubMed
- Burlet AJ, Jhanwar-Uniyal M, Chapleur-Chateau M, Burlet CR, Leibowitz SF ( 1992) Effect of food deprivation and refeeding on the concentration of vasopressin and oxytocin in discrete hypothalamic sites. Pharmacol Biochem Behav 43: 897–905. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical