Changes in intracellular calcium and glutathione in astrocytes as the primary mechanism of amyloid neurotoxicity - PubMed (original) (raw)
Changes in intracellular calcium and glutathione in astrocytes as the primary mechanism of amyloid neurotoxicity
Andrey Y Abramov et al. J Neurosci. 2003.
Abstract
Although the accumulation of the neurotoxic peptide beta amyloid (betaA) in the CNS is a hallmark of Alzheimer's disease, the mechanism of betaA neurotoxicity remains controversial. In cultures of mixed neurons and astrocytes, we found that both the full-length peptide betaA (1-42) and the neurotoxic fragment (25-35) caused sporadic cytoplasmic calcium [intracellular calcium ([Ca2+]c)] signals in astrocytes that continued for hours, whereas adjacent neurons were completely unaffected. Nevertheless, after 24 hr, although astrocyte cell death was marginally increased, approximately 50% of the neurons had died. The [Ca2+]c signal was entirely dependent on Ca2+ influx and was blocked by zinc and by clioquinol, a heavy-metal chelator that is neuroprotective in models of Alzheimer's disease. Neuronal death was associated with Ca2+-dependent glutathione depletion in both astrocytes and neurons. Thus, astrocytes appear to be the primary target of betaA, whereas the neurotoxicity reflects the neuronal dependence on astrocytes for antioxidant support.
Figures
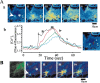
Figure 1.
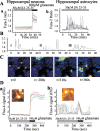
β Amyloid raises [Ca2+]c in astrocytes and not in neurons. A, Records of fura-2 fluorescence from neurons (a) and astrocytes (b) in hippocampal cocultures after exposure to βA25–35 peptide (50 μ
m
). The neurons showed no change in signal over a period of 35 min. Their identity was confirmed by their response to glutamate (100 μ
m
) at the end of this period. The astrocytes (b) showed complex [Ca2+]c fluctuations starting after ∼5–6 min of exposure to βA25–35 (50 μ
m
). These could continue for many hours. Some sample traces are extracted from this population and illustrated as Bi, Bii, and Biii. The images in C are taken from a time series of confocal images of a hippocampal coculture loaded with fluo-4. The field includes four neurons (n) surrounded by astrocytes. Once again, the astrocytes show complex transient and localized [Ca2+]c responses whereas the neurons show no change in signal at all. The traces in D originate from confocal images of a fluo-4-loaded hippocampal explant culture. Once again, neurons that showed a robust response to glutamate application showed no response to βA(a) whereas astrocytes showed complex [Ca2+]c fluctuations and only a small transient metabotropic [Ca2+]c response to glutamate (b). An image of a responding cell is inset for each signal.
Figure 2.
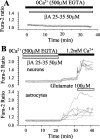
[Ca2+]c responses to βA are dependent on extracellular Ca2+. A, In the absence of external Ca2+, cortical astrocytes showed no change in [Ca2+]c after exposure to βA25–35 (50 μ
m
). B, In a coculture exposed to βA25–35 (50 μ
m
) in the absence of external Ca2+, no response was seen in either neurons or astrocytes.βA was then washed out and external Ca2+ added. Despite the removal of theβA, the addition of Ca2+ caused a large increase in [Ca2+]c in the astrocytes but only a very small change in the neurons, reflecting the restoration of basal calcium entry. Once again, the neuronal identity was confirmed by the response to 100 μ
m
glutamate at the end of the experiment.
Figure 3.
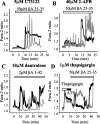
Intracellular Ca2+ stores do not make a significant contribution to the astrocyte [Ca2+]c response to βA. Manipulations that either block components of the IP3 and ryanodine signaling pathways or that empty ER stores do not significantly alter the astrocyte responses to βA. The application of 50 μ
m
βA25–35 caused [Ca2+]c transients in cortical astrocytes despite the presence of 5 μ
m
U73122 (an inhibitor of PLC; A), 40 μ
m
2-APB (B), dantrolene (an inhibitor of ryanodine receptors; C), and 1 μ
m
thapsigargin (an inhibitor of ER Ca2+ pumps; D), at a concentration that prevented the response to ATP (100 μ
m
).
Figure 4.
Mn 2+ quench confirms that astrocyte [Ca2+]c transients reflect transient Ca2+ influx. Fura-2-loaded hippocampal astrocytes showed typical [Ca2+]c fluctuations (black line) in response to 50 μ
m
βA25–35. A, In the absence of external Mn 2+, the fura-2 response excited at 360 nm (gray line) showed no change during the [Ca2+]c transients, confirming that this is close to the isosbestic [Ca2+]c-independent excitation wavelength for fura-2. Bi, Bii, With the addition of 40 μ
m
Mn 2+ each [Ca2+]c transient was accompanied by a step quench of the 360 nm fura-2 signal, confirming that each transient reflects a pulsed influx of divalent cations seen in response to βA25–35.
Figure 5.
Confocal imaging reveals focal Ca2+ influx in response to βA. In a hippocampal coculture loaded with fluo-4, confocal imaging during the exposure to βA shows that the change in [Ca2+]c can originate as a focal change that diffuses through the cell and may be restricted to the subplasmalemmal space. Aa, Time series of confocal images taken during a single [Ca2+]c transient response in an astrocyte. Note that the response begins with a focal rise in [Ca2+]c (arrowhead) followed by the slower spread through the cell. This is illustrated further in Ab, which shows a plot of the signal with time at four different locations in the cell (indicated color-coded on the inset image). The rapid rate of rise at the point of influx contrasts with the much slower increase seen deep in the cytosol of the cell. B, Series of images taken from another astrocyte during a response to βA25–35, again showing that the [Ca2+]c signal may be restricted to the periphery of the cell and fail to propagate through the cell. The first image of the sequence shows the raw data, where as the subsequent images show the ratio of the image sequence with respect to the first image of the sequence.
Figure 6.
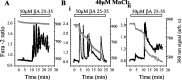
Responses to βA are blocked by Zn 2+ and clioquinol but not by Cu 2+. A, Addition of zinc (1 m
m
ZnCl2) suppressed the astrocyte response to βA. B, Addition of CuCl2 (100 μ
m
) had no apparent effect on the βA responses, but the heavy-metal chelator clioquinol (2 μ
m
) (C) suppressed the responses completely.
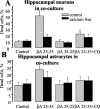
Figure 7.
βA causes Ca2+-dependent depletion of GSH in both neurons and astrocytes. MCB was used to image astrocyte and neuronal GSH by digital imaging. Hippocampal cocultures (15–20 DIV) (A, B) and cortical astrocyte cultures (C) were treated for 24 hr with βA25–35 or βA35–25 (50 μ
m
for both) and clioquinol (1 μ
m
) at 37°C in culture medium with (gray columns) or without (black columns) calcium. Mean intensities of MCB–GSH adduct fluorescence (arb.U) are presented. βA25–35 decreased GSH dramatically either in hippocampal astrocytes in coculture with neurons (A) or in cortical astrocytes in monoculture (C). The response was dependent on extracellular calcium and was also suppressed by clioquinol (C). βA35–25 had no effect. Note that neuronal GSH was also significantly reduced (B) and that the reduction was also calcium dependent, although it represents a proportionately smaller response than that of the astrocytes.
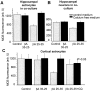
Figure 8.
βA causes Ca2+-dependent cell death in neurons and not in astrocytes. Effect of βA on viability of neurons and astrocytes. PI fluorescence was used to detect dead cells 24 hr after the addition of 50 μ
m
βA and 1 μ
m
clioquinol in the presence or absence of Ca2+. Dead cells were counted with respect to the total number of cells present, identified by staining nuclei with Hoechst 33342. βA caused a dramatic increase in cell death in neurons and only a modest increase in astrocyte cell death in hippocampal cocultures. Cell death was calcium dependent and cells were dramatically protected by 1 μ
m
clioquinol.
Similar articles
- Membrane cholesterol content plays a key role in the neurotoxicity of β-amyloid: implications for Alzheimer's disease.
Abramov AY, Ionov M, Pavlov E, Duchen MR. Abramov AY, et al. Aging Cell. 2011 Aug;10(4):595-603. doi: 10.1111/j.1474-9726.2011.00685.x. Epub 2011 Apr 11. Aging Cell. 2011. PMID: 21332922 - Mechanism of neuroprotection of melatonin against beta-amyloid neurotoxicity.
Ionov M, Burchell V, Klajnert B, Bryszewska M, Abramov AY. Ionov M, et al. Neuroscience. 2011 Apr 28;180:229-37. doi: 10.1016/j.neuroscience.2011.02.045. Epub 2011 Feb 24. Neuroscience. 2011. PMID: 21354274 - Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase.
Abramov AY, Canevari L, Duchen MR. Abramov AY, et al. J Neurosci. 2004 Jan 14;24(2):565-75. doi: 10.1523/JNEUROSCI.4042-03.2004. J Neurosci. 2004. PMID: 14724257 Free PMC article. - Calcium signals induced by amyloid beta peptide and their consequences in neurons and astrocytes in culture.
Abramov AY, Canevari L, Duchen MR. Abramov AY, et al. Biochim Biophys Acta. 2004 Dec 6;1742(1-3):81-7. doi: 10.1016/j.bbamcr.2004.09.006. Biochim Biophys Acta. 2004. PMID: 15590058 Review. - The role of an astrocytic NADPH oxidase in the neurotoxicity of amyloid beta peptides.
Abramov AY, Duchen MR. Abramov AY, et al. Philos Trans R Soc Lond B Biol Sci. 2005 Dec 29;360(1464):2309-14. doi: 10.1098/rstb.2005.1766. Philos Trans R Soc Lond B Biol Sci. 2005. PMID: 16321801 Free PMC article. Review.
Cited by
- Role of scavenger receptors in glia-mediated neuroinflammatory response associated with Alzheimer's disease.
Cornejo F, von Bernhardi R. Cornejo F, et al. Mediators Inflamm. 2013;2013:895651. doi: 10.1155/2013/895651. Epub 2013 May 7. Mediators Inflamm. 2013. PMID: 23737655 Free PMC article. Review. - Targeted polyphosphatase expression alters mitochondrial metabolism and inhibits calcium-dependent cell death.
Abramov AY, Fraley C, Diao CT, Winkfein R, Colicos MA, Duchen MR, French RJ, Pavlov E. Abramov AY, et al. Proc Natl Acad Sci U S A. 2007 Nov 13;104(46):18091-6. doi: 10.1073/pnas.0708959104. Epub 2007 Nov 6. Proc Natl Acad Sci U S A. 2007. PMID: 17986607 Free PMC article. - Mitochondrial Permeability Transition, Cell Death and Neurodegeneration.
Baev AY, Vinokurov AY, Potapova EV, Dunaev AV, Angelova PR, Abramov AY. Baev AY, et al. Cells. 2024 Apr 8;13(7):648. doi: 10.3390/cells13070648. Cells. 2024. PMID: 38607087 Free PMC article. Review. - Astrocytes respond to a neurotoxic Aβ fragment with state-dependent Ca2+ alteration and multiphasic transmitter release.
Pham C, Hérault K, Oheim M, Maldera S, Vialou V, Cauli B, Li D. Pham C, et al. Acta Neuropathol Commun. 2021 Mar 16;9(1):44. doi: 10.1186/s40478-021-01146-1. Acta Neuropathol Commun. 2021. PMID: 33726852 Free PMC article. - Calcium-Sensing Receptors of Human Neural Cells Play Crucial Roles in Alzheimer's Disease.
Chiarini A, Armato U, Liu D, Dal Prà I. Chiarini A, et al. Front Physiol. 2016 Apr 26;7:134. doi: 10.3389/fphys.2016.00134. eCollection 2016. Front Physiol. 2016. PMID: 27199760 Free PMC article. Review.
References
- Atwood CS, Moir RD, Huang X, Scarpa RC, Bacarra NM, Romano DM, Hartshorn MA, Tanzi RE, Bush AI ( 1998) Dramatic aggregation of Alzheimer Aβ by Cu(II) is induced by conditions representing physiological acidosis. J Biol Chem 273: 12817–12826. - PubMed
- Behl C, Davis JB, Lesley R, Schubert D ( 1994) Hydrogen peroxide mediates amyloid β protein toxicity. Cell 77: 817–822. - PubMed
- Blanchard BJ, Konopka G, Russell M, Ingram VM ( 1997) Mechanism and prevention of neurotoxicity caused by β-amyloid peptides: relation to Alzheimer's disease. Brain Res 776: 40–50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous