Acute remapping within the motor system induced by low-frequency repetitive transcranial magnetic stimulation - PubMed (original) (raw)
Clinical Trial
Acute remapping within the motor system induced by low-frequency repetitive transcranial magnetic stimulation
Lucy Lee et al. J Neurosci. 2003.
Abstract
Repetitive transcranial magnetic stimulation (rTMS) of human primary motor cortex (M1) changes cortical excitability at the site of stimulation and at distant sites without affecting simple motor performance. The aim of this study was to explore how rTMS changes regional excitability and how the motor system compensates for these changes. Using functional brain imaging, activation was mapped at rest and during freely selected finger movements after 30 min of 1 Hz rTMS. rTMS increased synaptic activity in the stimulated left M1 and induced widespread changes in activity throughout areas engaged by the task. In particular, movement-related activity in the premotor cortex of the nonstimulated hemisphere increased after 1 Hz rTMS. Analyses of effective connectivity confirmed that the stimulated part of M1 became less responsive to input from premotor and mesial motor areas. Conversely, after rTMS our results were consistent with increased coupling between an inferomedial portion of left M1 and anterior motor areas. These results are important for three reasons. First, they show changes in motor excitability to central inputs from other cortical areas (as opposed to peripheral or exogenous inputs used in previous studies). Second, they suggest that maintenance of task performance may involve activation of premotor areas contralateral to the site of rTMS, similar to that seen in stroke patients. Third, changes in motor activations at the site of rTMS suggest an rTMS-induced remodeling of motor representations during movement. This remapping may provide a neural substrate for acute compensatory plasticity of the motor system in response to focal lesions such as stroke.
Figures
Figure 1.
Experimental design. Subjects received 1 Hz real- or sham-rTMS on separate days. Changes in regional cerebral blood flow were mapped using PET. Six sequential H215O PET scans were acquired at baseline (B) or during the freely selected movement task (M) in alternation during the hour after the end of rTMS. The order of intervention (real-rTMS vs sham-rTMS) and experimental conditions were counterbalanced across subjects.
Figure 2.
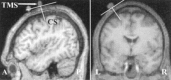
Position and orientation of TMS coil (TMS) relative to the central sulcus (CS) shown in one subject. N.B.: Capsules marking the position of the premotor cortex, visible in the sagittal scans, anterior to TMS, are part of a different experiment.
Figure 3.
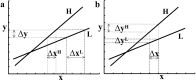
Interpretation of PPI analyses. This schematic shows that two mathematically equivalent but biologically complementary hypotheses can be used to motivate the same PPI. In both graphs, x represents activity in an index area subtending the physiological variance in the PPI analysis. Conditions H and L represent some psychological or experimental manipulation. In a during H, a unit increase in activity in area y (Δy) is associated with a small increase in activity in area x: Δx H (dotted line). During L, Δy is associated with a larger increase in activity in area x:Δx L(dashedline). Consequently, this is the change that one would predict if we thought area x was less responsive to Δy during H. In b during H, a unit increase in activity in area x (Δx) is associated with a large increase in activity in area y: Δy H (dotted line). During L, Δx is associated with a smaller change in activity in area y: Δy L (dashed line). In short, exactly the same PPI would be predicted if we thought that area y was more responsive to Δx during H.
Figure 7.
Changes in effective connectivity (psychophysiological interaction) with the site of rTMS stimulation. Top left panel, Areas showing positive PPI with the site of rCBF increase in left sensorimotor cortex after rTMS. Results are displayed as statistical parametric maps in sagittal, coronal, and transverse projections in stereotactic space. The grayscale areas show all significant voxels at p < 0.001, uncorrected. The black circle shows the location of the region of interest used as the physiological variate in the interaction. The design matrix is displayed alongside the statistical parametric maps. Graphical representations illustrate the psychophysiological interactions between the site of rTMS region of interest (x = -30, y = -26, z = 62) (abscissa) and significant areas. Regression lines between the activity in the two regions have been fitted: sham-rTMS = S (triangles) and real-rTMS = R (diamonds). i, Left dorsal premotor area (x = -14, y = -6, z = 66). Sham-rTMS: _r_2 = 0.04, F = 2.01, gradient = 0.01; real-rTMS: _r_2 = 0.63, F = 79.40, gradient = 0.80. ii, Proximate left sensorimotor region (x = -38, y = -20, z = 46). Sham-rTMS: _r_2 = 0.22, F = 13.19, gradient = 0.36; real-rTMS: _r_2 = 0.66 F = 87.37, gradient = 0.81. iii, Left mesial motor area (x = -4, y = 14, z = 34). Sham-rTMS: _r_2 = 0.00, F = 2.01, gradient = 0.01; real-rTMS: _r_2 = 0.40 F = 30.17, gradient = 0.50.
Figure 4.
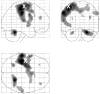
Regional activations during freely selected finger movements (main effect of movement). Results are displayed as statistical parametric maps on sagittal, coronal, and transverse projections in stereotactic space. The grayscale areas indicate all significant voxels showing a movement-related activation at p < 0.05 (corrected for multiple comparisons). The X indicates the site of rTMS stimulation.
Figure 5.
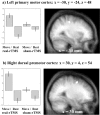
Regional increases in rCBF after rTMS to the left motor cortex (main effect of rTMS). Left primary motor and bilateral increases in rCBF displayed on an axial section of averaged anatomical MRI scans. Results are displayed at p < 0.001 uncorrected, masked by main effect of movement, p < 0.001 uncorrected (because these effects are orthogonal this corresponds to p < 0.00001). Parameter estimates showing mean (±SE) activation during the four experimental conditions are also displayed.
Figure 6.
Areas of the brain showing differential movement-related responses after rTMS (interaction between movement and rTMS). Results are displayed on sagittal sections of averaged anatomical MRI scans at p < 0.001 uncorrected, masked by main effect of movement as for Figure 5. Localization of activation and parameter estimates for left sensorimotor site (a) and right premotor site (b).
Figure 8.
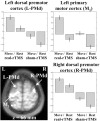
Changes in effective connectivity (psychophysiological interaction) with the movement-related activations. a, Areas showing positive PPI with left sensorimotor region of interest (x = -42, y = -26, z = 56) during movement-related activity after real-rTMS compared with sham-rTMS, displayed as described for Figure 7. b, Graphical representation illustrating the psychophysiological interactions between left sensorimotor region of interest (abscissa) and a proximate left sensorimotor region (x = -38, y = -20, z = 46). Regressions lines between the activity in the two regions have been fitted: sham-rTMS = S (squares) (_r_2 = 0.36, F = 25.68, gradient = 0.37) and real-rTMS = R (diamonds) (_r_2 = 0.79, F = 170.87, gradient = 0.71). c, Areas showing positive PPI with left premotor region of interest (x = -26, y = -14, z = 68), as described in Figure 7. d, Graphical representations illustrating the psychophysiological interactions between left premotor region of interest (abscissa) and left sensorimotor region (x = -36, y = -22, z = 44), as described for b. Sham-rTMS: _r_2 = 0.06, F = 2.66, gradient = 0.11; real-rTMS: _r_2 = 0.43, F = 34.13, gradient = 0.78. e, Areas showing positive PPI with the left SMA region of interest (x = -12, y = -4, z = 56), as described for Figure 7. f, Graphical representation illustrating the psychophysiological interactions between left SMA region of interest (abscissa) and left sensorimotor region (x = -30, y = -26, z = 54), as described for b. Sham-rTMS: _r_2 = 0.25, F = 15.52, gradient = 0.43; real-rTMS: _r_2 = 0.61, F = 71.0, gradient = 1.07.
Figure 9.
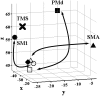
Three-dimensional representation of the relative positions of the primary motor cortex sites identified in the three PPI analyses shown in Figure 8_a_—f. The solid circle, square, and triangle symbols represent the regions of interest (the maxima of the main effect of movement) from which activity was used to create the PPI (see Materials and Methods). The open circle, square, and triangle symbols indicate the relative position of the sites in primary motor cortex that are more strongly influenced by activity in SM1, PMd, and SMA, respectively, after rTMS. The solid diamond indicates the position of the SM1 site seen in the movement-by-rTMS interaction (Fig. 6). X marks the site of stimulation with 1 Hz rTMS.
Similar articles
- Frequency specific changes in regional cerebral blood flow and motor system connectivity following rTMS to the primary motor cortex.
Rounis E, Lee L, Siebner HR, Rowe JB, Friston KJ, Rothwell JC, Frackowiak RS. Rounis E, et al. Neuroimage. 2005 May 15;26(1):164-76. doi: 10.1016/j.neuroimage.2005.01.037. Neuroimage. 2005. PMID: 15862216 Clinical Trial. - Inducing homeostatic-like plasticity in human motor cortex through converging corticocortical inputs.
Pötter-Nerger M, Fischer S, Mastroeni C, Groppa S, Deuschl G, Volkmann J, Quartarone A, Münchau A, Siebner HR. Pötter-Nerger M, et al. J Neurophysiol. 2009 Dec;102(6):3180-90. doi: 10.1152/jn.91046.2008. Epub 2009 Sep 2. J Neurophysiol. 2009. PMID: 19726723 - Neural substrates of low-frequency repetitive transcranial magnetic stimulation during movement in healthy subjects and acute stroke patients. A PET study.
Conchou F, Loubinoux I, Castel-Lacanal E, Le Tinnier A, Gerdelat-Mas A, Faure-Marie N, Gros H, Thalamas C, Calvas F, Berry I, Chollet F, Simonetta Moreau M. Conchou F, et al. Hum Brain Mapp. 2009 Aug;30(8):2542-57. doi: 10.1002/hbm.20690. Hum Brain Mapp. 2009. PMID: 19072894 Free PMC article. - Intensity-dependent regional cerebral blood flow during 1-Hz repetitive transcranial magnetic stimulation (rTMS) in healthy volunteers studied with H215O positron emission tomography: I. Effects of primary motor cortex rTMS.
Speer AM, Willis MW, Herscovitch P, Daube-Witherspoon M, Shelton JR, Benson BE, Post RM, Wassermann EM. Speer AM, et al. Biol Psychiatry. 2003 Oct 15;54(8):818-25. doi: 10.1016/s0006-3223(03)00002-7. Biol Psychiatry. 2003. PMID: 14550681 Review. - Non-Invasive Brain Stimulation to Enhance Post-Stroke Recovery.
Kubis N. Kubis N. Front Neural Circuits. 2016 Jul 27;10:56. doi: 10.3389/fncir.2016.00056. eCollection 2016. Front Neural Circuits. 2016. PMID: 27512367 Free PMC article. Review.
Cited by
- Cortical activation changes underlying stimulation-induced behavioural gains in chronic stroke.
Stagg CJ, Bachtiar V, O'Shea J, Allman C, Bosnell RA, Kischka U, Matthews PM, Johansen-Berg H. Stagg CJ, et al. Brain. 2012 Jan;135(Pt 1):276-84. doi: 10.1093/brain/awr313. Epub 2011 Dec 6. Brain. 2012. PMID: 22155982 Free PMC article. - Entropy Analysis of High-Definition Transcranial Electric Stimulation Effects on EEG Dynamics.
Nascimento DC, Depetri G, Stefano LH, Anacleto O, Leite JP, Edwards DJ, Santos TEG, Louzada Neto F. Nascimento DC, et al. Brain Sci. 2019 Aug 20;9(8):208. doi: 10.3390/brainsci9080208. Brain Sci. 2019. PMID: 31434225 Free PMC article. - Time-specific contribution of the supplementary motor area to intermanual transfer of procedural knowledge.
Perez MA, Tanaka S, Wise SP, Willingham DT, Cohen LG. Perez MA, et al. J Neurosci. 2008 Sep 24;28(39):9664-9. doi: 10.1523/JNEUROSCI.3416-08.2008. J Neurosci. 2008. PMID: 18815252 Free PMC article. - Primary somatosensory contribution to action observation brain activity-combining fMRI and cTBS.
Valchev N, Gazzola V, Avenanti A, Keysers C. Valchev N, et al. Soc Cogn Affect Neurosci. 2016 Aug;11(8):1205-17. doi: 10.1093/scan/nsw029. Epub 2016 Mar 15. Soc Cogn Affect Neurosci. 2016. PMID: 26979966 Free PMC article. - Modulation of cortical oscillatory activity during transcranial magnetic stimulation.
Brignani D, Manganotti P, Rossini PM, Miniussi C. Brignani D, et al. Hum Brain Mapp. 2008 May;29(5):603-12. doi: 10.1002/hbm.20423. Hum Brain Mapp. 2008. PMID: 17557296 Free PMC article.
References
- Abbott LF, Varela JA, Sen K, Nelson SB ( 1997) Synaptic depression and cortical gain control. Science 275: 220–224. - PubMed
- Bohning DE, Shastri A, McGavin L, McConnell KA, Nahas Z, Lorberbaum JP, Roberts DR, George MS ( 2000) Motor cortex brain activity induced by 1-Hz transcranial magnetic stimulation is similar in location and level to that for volitional movement. Invest Radiol 35: 676–683. - PubMed
- Buonomano DV, Merzenich MM ( 1998) Cortical plasticity: from synapses to maps. Annu Rev Neurosci 21: 149–186. - PubMed
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG ( 1997) Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology 48: 1398–1403. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical